Cardiac dysfunction is common in patients with thalassaemia and is the leading cause of mortality in adult patients. Transfusional iron overload can affect heart function by directly damaging tissue through iron deposition or via iron-mediated effects at other sites. The main cardiac abnormalities reported in patients with thalassaemia and iron overload are left ventricular systolic and diastolic dysfunction, pulmonary hypertension, valvulopathies, arrhythmias and pericarditis. Prevalence varies according to the type of thalassaemia. However, even though patients with thalassaemia intermedia require fewer transfusions than those with thalassaemia major, they are still at high risk for cardiac complications. With the introduction of new technologies such as cardiac magnetic resonance T2*, the early detection of cardiac iron overload and associated cardiac dysfunction is now possible, allowing time for reversal through iron chelation therapy. Although chelation therapy can reverse iron-mediated cardiac disease by removing iron from iron-loaded cardiomyocytes and by alleviating the systemic iron overload contributing to heart failure, the challenges of deferoxamine infusions can significantly impact on compliance and, therefore, prognosis. The introduction of new oral iron chelators, together with improved understanding of the mechanisms and consequences of transfusional iron overload, should allow the continued improvement in cardiac outcomes for patients with thalassaemia and other transfusion-dependent anaemias.
Introduction
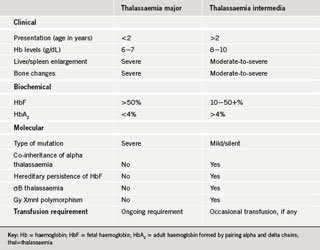
Thalassaemia was first described as a significant problem in the UK in the mid-1950s and was predominantly observed in patients of African or Mediterranean descent. By 1998, more than 800 patients with thalassaemia major were included on the UK Thalassaemia Register.1 As with thalassaemia major, patients with thalassaemia intermedia have a homozygous genotype (i.e. have inherited an affected beta-gene from both parents) but they demonstrate milder clinical symptoms. The clinical phenotypes of thalassaemia intermedia lie between those of thalassaemia minor and major. Although there is substantial clinical overlap between the conditions, some key points of differentiation have been identified (table 1).
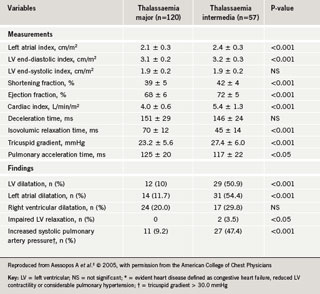
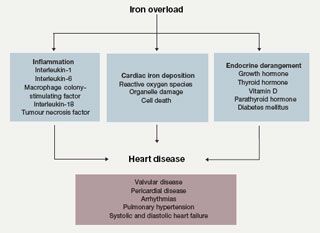
Cardiac dysfunction with pump failure and sudden cardiac death are the leading causes of death in adult thalassaemia patients; even today, the mortality rate for patients with thalassaemia major remains 50% before the age of 35 years. Iron overload from repeated blood transfusions affects heart function by directly damaging tissue through iron deposition in cardiac cells, as well as iron-mediated effects on vascular and endocrine functions. The cardiac abnormalities reported in iron-overloaded patients with thalassaemia encompass left ventricular (LV) systolic and diastolic dysfunction, pulmonary hypertension, valvulopathies, arrhythmias and pericarditis, with varying prevalence according to thalassaemia type (table 2). These cardiac abnormalities are as a consequence of the general co-morbid conditions in thalassaemia but are closely related to concomitant endocrine deficiencies, hypercoagulability state and inflammatory milieu (figure 1). Iron chelation therapy aims to reverse iron-mediated cardiac disease by removing iron from iron-loaded cardiomyocytes, and by alleviating the systemic iron overload that underlies accessory factors contributing to heart failure.
Deposition of iron in the heart
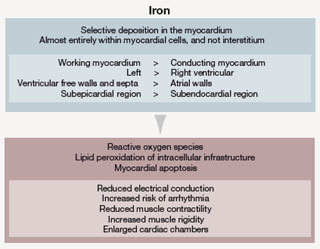
In patients with thalassaemia major, ineffective erythropoiesis results in a dramatic increase in plasma iron turnover, 10–15 times greater than that in normal subjects, with concomitant increase in non-transferrin-bound iron (NTBI) levels. Plasma NTBI is thought to play a major role in iron loading of the heart, probably through adventitious uptake by L-type voltage-dependent Ca2+ channels. Iron is deposited in the heart in a heterogeneous pattern, with working cardiomyocytes accumulating more iron than fibroblasts (figure 2). Iron’s toxicity within cells arises from its capacity to catalyse the production of reactive oxygen species that cause lipid peroxidation and organelle damage, which lead ultimately to cell death and fibrosis. In the heart, these effects reduce muscle contractility and electrical conduction, and lead to structural changes such as enlargement of cardiac chambers and muscle rigidity (figure 2).
Assessing cardiac iron load
Previously, the only way to measure cardiac iron load was by biopsy. This invasive technique can result in complications, and because iron is distributed heterogeneously in the heart, may not provide a true estimate of overall iron concentration. A more complete picture of cardiac iron load can be obtained non-invasively using T2* cardiac magnetic resonance imaging (MRI). This technique measures the time taken for protons to relax back to their basal energy level after excitation by a pulse of electromagnetic radiation. T2* values <20 ms indicate abnormal cardiac iron loading, and values <8 ms indicate a serious cardiac iron burden.3 Recent data have demonstrated correlation between T2* values, ejection fraction, and clinical status, as evidenced by reversal of heart failure with therapy.3,4 Moreover, cardiac MRI permits periodic monitoring to assess changes in cardiac iron levels, and can be used in parallel with other techniques to assess cardiac function. However, outside of Europe and North America MRI is not widely used due to the limited availability of scanners and the high cost, particularly in developing countries. Efforts are currently being made to increase the accessibility of MRI worldwide.
Diastolic dysfunction
Many studies have described a late restrictive filling pattern in patients with thalassaemia major, with a prevalence of 37.6% in a Greek cohort.5 Some authors have suggested that the earliest sign of diastolic dysfunction is
prolongation of the isovolumic relaxation time.6 However, not only are data on early diastolic findings conflicting, there is also no comprehensive prospective study showing how diastolic dysfunction occurs and which prognostic indicators are related which to its development.7 In addition, accurate identification of diastolic dysfunction depends on the interpretation of numerous Doppler parameters. Anaemia itself, which causes a decrease in blood viscosity and peripheral systemic vascular resistance, remains a confounding factor in assessing cardiac dysfunction. However, tissue Doppler parameters, although not totally preload- or anaemia-independent, may be less subject to variation and require more research in the future. Kremastinos et al. highlighted the importance of preload dependency, by suggesting that even serum ferritin levels have no predictive effect on diastolic dysfunction beyond that caused by anaemia.8
Pulmonary hypertension and right ventricular dysfunction
Pulmonary hypertension and right ventricular dysfunction are important components of cardiac dysfunction in thalassaemia. With improvements in the treatment of thalassaemia major, pulmonary hypertension has become the primary cardiopathy in thalassaemia intermedia (17–60%, depending on ethnicity). It is now thought that right ventricular dysfunction occurs independently of pulmonary hypertension.9 Atichartakarn et al. suggested that pulmonary hypertension is unrelated to pulmonary embolisation,10 but is secondary to increased pulmonary vascular resistance due to a chronic low-grade hypercoagulable condition, as proposed previously.11 This interpretation is supported by necropsy findings from thalassaemia patients showing pulmonary artery obstruction without apparent deep vein thrombosis.12 Pulmonary hypertension may be reversible with anaemia correction, iron chelation therapy, aspirin use and anticoagulation with warfarin.13 The use of sildenafil has been reported with success in case reports, but further clinical evaluation is required before a recommendation can be made.14 Blood transfusions to prevent pulmonary hypertension in thalassaemia intermedia are currently a subject of much debate.
Inflammation, congestive heart failure and thalassaemia
There is increasing evidence supporting a role for inflammatory mediators in congestive heart failure. Many reports have demonstrated an increase in tumour necrosis factor alpha, interleukin (IL)-1, IL-6 and IL-18, and chemokines in patients with congestive heart failure, with levels increasing according to disease severity.15-17 These inflammatory mediators are increased within the failing myocardium as well as in the circulation.18 A similar inflammatory cytokine profile has been found in thalassaemia major patients, although evidence of a reversal in this profile has been observed in iron-overloaded patients after treatment with chelation therapy.19,20 Demonstrating that the high levels of cytokines in iron-overloaded thalassaemia major patients contribute to the development of congestive heart failure is an intriguing hypothesis subject to ongoing research.
Pericarditis and valvulopathy
Pericarditis is a well-known cardiac complication in thalassaemia. Chronic pericardial changes, small-to-moderate pericardial effusion and pericardial thickening have been detected by echocardiography, even in patients without a history of pericarditis.21 A pseudoxanthoma elasticum equivalent has been described in sickling syndromes and beta-thalassaemia, particularly in thalassaemia intermedia patients over 30 years of age.22 Iron overload, unbound fractions of haemoglobin, and plasma membrane particles leading to oxidative damage of the elastic tissue are suggested to cause pseudoxanthoma elasticum equivalent in the various haemoglobinopathies.23,24 Pseudoxanthoma elasticum is thought to be the common pathogenic basis for the higher prevalence of leaflet thickening, endocardial calcification, valvular regurgitation and rupture of chordae tendinae in beta-thalassaemia.23 Aessopos et al. have proposed that these modifications are probably caused by the volume overload and cardiac dilatation.21 They detected mitral valve prolapse in only 5.4% of patients with thalassaemia intermedia, a prevalence comparable to that of healthy controls.
Endocrine abnormalities and congestive heart failure
Thalassaemia and iron overload are associated with several endocrinopathies, and the effect of these on the heart cannot be disregarded. In one UK-based study, two-thirds of thalassaemia patients had at least one endocrine disorder while 40% had multiple endocrinopathies.25 For patients with growth hormone deficiency, replacement therapy with recombinant growth hormone led to a significant increase in cardiac output and a parallel decrease in systemic vascular resistance.26 Nevertheless, there is currently no consensus as to who or when to screen for growth hormone deficiency, and no survival benefit has yet been demonstrated with replacement therapy. Diabetes mellitus is a recognised complication of iron overload in thalassaemia due to iron-mediated destruction of beta cells,27 and it is a well established risk factor for cardiovascular disease. Considering the role played by diabetes mellitus in endothelial inflammation, which is an independent risk factor for survival in patients with congestive heart failure, thalassaemic patients with diabetes mellitus could be at increased risk of cardiovascular disease. Hypoparathyroidism has a reported prevalence of 7.6–13.5% in thalassaemic patients (12.5% in the UK).28 Together with a potential effect on the heart through hypocalcaemia, it is likely that endocrine disorders are a major contributing factor for decompensated heart failure in thalassaemia.
Arrhythmogenicity
Arrhythmias and sudden cardiac death remain a serious concern in thalassaemia. Haemochromatic arrhythmias include heart block, atrial and ventricular extrasystoles, and atrial and ventricular tachycardia. These arrhythmias are a therapeutic challenge, being secondary to the heterogeneous pattern of iron deposition in the heart.29 They respond favourably to intensive chelation therapy, whereas antiarrhythmic drugs should be used with caution because of side effects (e.g. amiodarone may contribute to thyroid dysfunction and digoxin may have pro-arrhythmic potential). Furthermore, Franzoni et al. found that patients with thalassaemia major have reduced heart rate variability and an increased prevalence of ventricular late potentials (31.5%), associated with some non-sustained ventricular tachycardia.30 These findings are contrary to a recent report by Isma’eel et al., which demonstrated a 10 times lower prevalence of ventricular late potentials (3%) among Lebanese patients with thalassaemia major.31 This difference may be due to ethnic variation or cut-off points for the specific measures used in the former study, in accordance with the American Standards.30
Cardiac dysfunction in non-thalassaemia patients
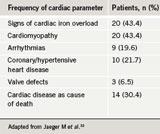
Cardiac dysfunction is less common and not as clearly defined in patients with transfusion-dependent anaemias other than thalassaemia, although a few studies have been published. One study evaluated cardiac function in 46 multi-transfused patients with various low-risk myelodysplastic syndromes. Of these patients, more than 40% had evidence of secondary haemochromatosis; the heart was the most commonly affected organ (table 3).32 Another study enrolled 15 regularly transfused non-thalassaemia patients; diagnoses included sideroblastic anaemia (n=5), pure red cell aplasia (n=3), myelofibrosis, hypoplastic anaemia, refractory anaemia (n=2 for all) and aplastic anaemia (n=1).33 Clinical evidence of congestive heart failure was observed in four patients (26.7%), all of whom also had symptomatic coronary artery disease. Increased LV end-diastolic volume was observed in seven patients (46.7%), five of whom also had increased LV end-systolic volume. Two of the most heavily
transfused patients had no evidence of LV dysfunction, but one had clinically compensated congestive heart failure and the other died of intractable congestive heart failure within six months of the study.
Iron chelation therapy and management of heart failure in thalassaemia
In terms of reversing heart disease, iron chelation therapy has a number of aims: to remove iron from cardiac cells and from other cells that exert effects on the heart (e.g. endocrine cells); to prevent further accumulation of organ iron by limiting ongoing exposure to NTBI; to restrict the ability of iron in the heart to catalyse oxidative damage. Deferoxamine (DFO) has been the only chelating agent available to most patients over the last four decades. While DFO remains the most widely used agent, it is well established that poor outcomes are due to suboptimal compliance with its demanding infusion regimen. It is hoped that the oral agents deferiprone and deferasirox, which has been recently introduced in the UK, will improve the long-term compliance.
There is good evidence that iron chelation can reduce cardiac iron burden and reverse iron-mediated cardiac disease. Continuous DFO (24 hours a day, seven days a week) produced a progressive reduction in cardiac iron load and improvement in cardiac function in patients with severe transfusional iron overload and symptoms of cardiac dysfunction.34 The newer, oral chelators appear to be more efficient than DFO at removing cardiac iron. A one-year randomised controlled trial (n=61) found that deferiprone was more effective than DFO at reducing cardiac iron in patients with mild cardiac iron load, and without symptoms of heart failure.35 In a subset of patients (n=30) assessed by cardiac MRI in a large one-year randomised controlled trial, deferasirox produced a statistically significant reduction in cardiac iron load that was greater than the reduction observed with DFO.36 This superiority may result from improved access of chelator into cardiomyocytes, together with continuous exposure to chelator to limit ongoing uptake of iron.37,38 Results of trials are awaited to confirm the beneficial cardiac effects of deferasirox.
The efficacy of combination therapy with DFO and deferiprone for the removal of cardiac iron has also been evaluated. Origa et al. observed a significant improvement in LV ejection fraction over 12–57 months of treatment with continuous deferiprone and intermittent administration of subcutaneous DFO.39 A study using the recommended regimen of the International Committee on Oral Chelators (deferiprone 80–110 mg/kg/day every day and DFO 40–60 mg/kg at least three days/week for either 8–12 hours using a pump or for 24 hours using an infuser), observed normalisation of cardiac T2* in all patients; in two patients with severe and moderate cardiac iron burden, normalisation was achieved within 9–10 months.40
The pharmacological management of congestive heart failure (angiotensin-converting enzyme [ACE] inhibitors and beta blockers) in thalassaemia is predominantly based on extrapolation from the general heart failure literature. With the exception of enalapril, which has been shown to improve LV systolic and diastolic function in transfusion-dependent patients with thalassaemia major, there is no real evidence supporting the use of commonly prescribed heart failure therapies (e.g. spironolactone, beta blockers and digoxin) specifically in iron overload.
Conclusions
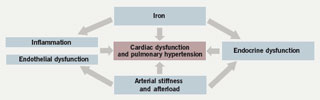
Iron overload in patients with thalassaemia and other anaemias impacts on cardiac function directly through cardiac iron loading, and indirectly through iron-mediated effects at other sites (figure 3). Patients with thalassaemia intermedia who require fewer transfusions than patients with thalassaemia major are still at high risk for cardiac complications, albeit with some differences in clinical manifestation.21 Congestive heart failure in patients with thalassaemia is a dynamic process involving inflammation and endocrine dysfunctions that affect the whole cardiovascular system. With the introduction of T2* cardiac MRI, it is now feasible to detect cardiac iron overload and associated subclinical cardiac dysfunction at an early stage, allowing time for reversal through iron chelation treatment. The introduction of oral iron chelators such as deferasirox, together with improved understanding of the mechanisms and consequences of transfusional iron overload, may presage a continuing improvement in cardiac outcomes for patients with thalassaemia in decades to come.
Conflict of interest
AT and MC are members of the Novartis Speakers’ Bureau. HI: none declared.
Key messages
- Cardiac dysfunction is the leading cause of death in adult patients with thalassaemia
- Transfusional iron overload can impact heart function directly or by other iron-mediated effects
- Early detection of cardiac iron load is now possible with emerging technologies such as cardiac magnetic resonance imaging (MRI) T2*
- Iron chelation therapy can reverse iron-mediated cardiac disease
References
- Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355:2051–2.
- Aessopos A, Farmakis D, Deftereos S et al. Thalassaemia heart disease: a comparative evaluation of thalassaemia major and thalassaemia intermedia.Chest 2005;127: 1523–30.
- Anderson LJ, Holden S, Davis B et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22:2171–9.
- Pennell DJ. T2* magnetic resonance and myocardial iron in thalassaemia. Ann N Y Acad Sci 2005;1054:373–8.
- Aessopos A, Farmakis D, Hatziliami A et al. Cardiac status in well-treated patients with thalassaemia major. Eur J Haematol 2004;73:359–66.
- Gharzuddine WS, Kazma HK, Nuwayhid IA et al. Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxation. Eur J Echocardiogr 2002;3:47–51.
- Bosi G, Crepaz R, Gamberini MR et al. Left ventricular remodelling, and systolic and diastolic function in young adults with beta thalassaemia major: a Doppler echocardiographic assessment and correlation with haematological data. Heart 2003;89:762–6.
- Kremastinos DT, Tsiapras DP, Tsetsos GA et al. Left ventricular diastolic Doppler characteristics in beta-thalassaemia major. Circulation 1993;88:1127–35.
- Hahalis G, Manolis AS, Apostolopoulos D et al. Right ventricular cardiomyopathy in beta-thalassaemia major. Eur Heart J 2002;23:147–56.
- Atichartakarn V, Likittanasombat K, Chuncharunee S et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol 2003;78:139–45.
- Rostagno C, Prisco D, Abbate R et al. Pulmonary hypertension associated with long-standing thrombocytosis. Chest 1991;99:1303–05.
- Sonakul D, Fucharoen S. Pulmonary thromboembolism in thalassemic patients. Southeast Asian J Trop Med Public Health 1992;23(suppl 2):25–8.
- Atichartakarn V, Chuncharunee S, Chandanamattha P et al. Correction of hypercoagulability and amelioration of pulmonary arterial hypertension by chronic blood transfusion in an asplenic hemoglobin E/beta-thalassaemia patient. Blood 2004;103:2844–6.
- Littera R, La Nasa G, Derchi G et al. Long-term treatment with sildenafil in a thalassemic patient with pulmonary hypertension. Blood 2002;100:1516–17.
- Aukrust P, Ueland T, Lien E et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1999;83:376–82.
- Levine B, Kalman J, Mayer L et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–41.
- Testa M, Yeh M, Lee P et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol 1996;28:964–71.
- Torre-Amione G, Kapadia S, Lee J et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation1996;93:704–11.
- Del Vecchio GC, Schettini F, Piacente L et al. Effects of deferiprone on immune status and cytokine pattern in thalassaemia major. Acta Haematol2002;108:144–9.
- Aggeli C, Antoniades C, Cosma C et al. Endothelial dysfunction and inflammatory process in transfusion-dependent patients with beta-thalassaemia major. Int J Cardiol 2005;105:80–4.
- Aessopos A, Farmakis D, Karagiorga M et al. Cardiac involvement in thalassaemia intermedia: a multicenter study. Blood 2001;97:3411–16.
- Aessopos A, Savvides P, Stamatelos G et al. Pseudoxanthoma elasticum-like skin lesions and angioid streaks in beta-thalassaemia. Am J Hematol1992;41:159–64.
- Farmakis D, Deftereos S, Giakoumis A et al. Rupture of chordae tendineae in patients with beta-thalassaemia. Eur J Haematol 2004;72:296–8.
- Aessopos A, Farmakis D, Loukopoulos D. Elastic tissue abnormalities in inherited haemolytic syndromes. Eur J Clin Invest 2002;32:640–2.
- Jensen CE, Tuck SM, Old J et al. Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with beta-thalassaemia. Eur J Haematol 1997;59:76–81.
- Boger RH, Skamira C, Bode-Boger SM et al. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 1996;98: 2706–13.
- Mohammadian S, Bazrafshan HR, Sadeghi-Nejad A. Endocrine gland abnormalities in thalassaemia major: a brief review. J Pediatr Endocrinol Metab2003;16:957–64.
- Telfer PT, Prestcott E, Holden S et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol 2000;110:971–7.
- Laurita KR, Chuck ET, Yang T et al. Optical mapping reveals conduction slowing and impulse block in iron-overload cardiomyopathy. J Lab Clin Med2003;142:83–9.
- Franzoni F, Galetta F, Di Muro C et al. Heart rate variability and ventricular late potentials in beta-thalassaemia major. Haematologica 2004;89:233–4.
- Isma’eel H, Taher A, Shamseddeen W et al. SAECG parameters and left ventricular chamber sizes: lesson from anemia conditions in thalassaemia major patients. Int J Cardiol 2006;113:E102–E104.
- Jaeger M, Aul C, Sohngen D et al. Iron overload in polytransfused patients with MDS: use of L1 for oral iron chelation. Drugs of Today 1992;28(suppl A):143–7.
- Schafer AI, Cheron RG, Dluhy R et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med 1981;304:319–24.
- Anderson LJ, Westwood MA, Holden S et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol 2004;127:348–55.
- Pennell DJ, Berdoukas V, Karagiorga M et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassaemia major patients with asymptomatic myocardial siderosis. Blood 2006;107:3738–44.
- Porter JB, Tanner MA, Pennell DJ et al. Improved myocardial T2* in transfusion dependent anemias receiving ICL670 (deferasirox). Blood2005;106(11):abstract 3600.
- Glickstein H, Ben El R, Shvartsman M et al. Intracellular labile iron pools as direct targets of iron chelators. A fluorescence study of chelator action in living cells. Blood 2005;106:3242–50.
- Nisbet-Brown E, Olivieri NF, Giardina PJ et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2003;361:1597–602.
- Origa R, Bina P, Agus A et al. Combined therapy with deferiprone and desferrioxamine in thalassaemia major. Haematologica 2005;90:1309–14.
- Kolnagou A, Kontoghiorghes GJ. Effective combination therapy of deferiprone and deferoxamine for the rapid clearance of excess cardiac iron and the prevention of heart disease in thalassaemia. The Protocol of the International Committee on Oral Chelators. Hemoglobin 2006;30:239–49.
