Cardiac disease remains the commonest cause of maternal death in the UK. While some deaths are unavoidable, pre-pregnancy counselling for women with acquired or congenital heart disease is important and counselling should be viewed as the mainstay of clinical practice. It provides women with information about the risk a pregnancy may pose to their health and to that of a foetus and provides an opportunity for an up-to-date assessment of the cardiac condition and a medication review. All cardiologists should recognise the need to raise the issue of pregnancy whenever a diagnosis of acquired heart disease is made in a woman of childbearing age. Although women with heart disease are at increased risk during pregnancy, the majority of women will have a good outcome with careful management.
Introduction

Women with cardiac disease are at increased risk during pregnancy due, in part, to the volume loading and increased cardiac output associated with normal pregnancy. Any haemodynamic changes are magnified in the case of a multiple pregnancy. It is essential that any woman with acquired or congenital heart disease, or those at increased risk, e.g. an adult survivor of childhood cancer who may have subclinical left ventricular dysfunction, be seen for pre-pregnancy counselling to quantify their risk and optimise their cardiac state prior to conception.
Deaths from cardiac disease in pregnancy are increasing, and cardiac disease remains the most common cause of maternal death in the UK.1 The last confidential enquiry into maternal deaths, once again, highlighted the issue that many women who died from pre-existing disease, failed to receive pre-pregnancy counselling or advice. Although some women who die have occult disease, unmasked by the haemodynamic burden of pregnancy, and others, e.g. spontaneous coronary artery dissection, are unavoidable, there are many women who have a number of risk factors that place them at increased risk, but who are not assessed pre-pregnancy, or the severity of their cardiac situation, in the context of a pregnancy, has not been recognised. Most deaths in pregnancy occur in women with acquired rather than congenital heart disease, but both are associated with significant morbidity. Furthermore, a salient point from the last confidential enquiry into maternal deaths was the importance of excess maternal weight. Sixty per cent of women, who died from cardiac disease with a documented weight, were overweight or obese. Women with cardiac disease may also be at increased risk of adverse obstetric and foetal outcome due to their cardiac condition.
Role of pre-pregnancy counselling
Pre-pregnancy counselling should provide women with information about the risk a potential pregnancy may pose to their health and their foetus. This includes:
- deterioration of functional class in pregnancy, which may not recover post-partum
- risk of heart failure
- thromboembolic risk
- arrhythmia
- increased risk of non-cardiac events, e.g. pre-eclampsia
- potential for their cardiac status to negatively impact on the foetus, e.g. preterm birth and/or foetal growth restriction
- medication alteration
- anticoagulation requirements and strategy during pregnancy.
The counselling should be non-directive, ideally involving the prospective father and, if an interpreter is required, a professional interpreter rather than a family member should be used. The counselling provides an opportunity for an up-to-date assessment of a woman’s cardiac condition and review of current medication, giving advice on changes that will be required in the event of pregnancy, e.g. cessation of angiotensin-converting enzyme (ACE) inhibitors in the event of a positive pregnancy test. Medication review is most important for anticoagulation strategies for mechanical valve replacements. Pre-pregnancy assessment permits any risk-lowering, or prognostically significant, intervention to be carried out prior to conception. It is also an opportunity to give general advice about lowering risk if pregnancy is intended, e.g. optimising weight, smoking cessation advice, having a good diet, taking extra folic acid, checking rubella status (and being vaccinated if not immune) and discussing the level of expected medical input during a future pregnancy. In some women, discussion about the risk of inheritance is appropriate, and referral to a geneticist may be required. Pre-pregnancy counselling may include a discussion about the long-term outlook for the woman in regard to her cardiac condition – in other words, her life expectancy, and the possibility of her needing major surgery while her children are still young. Good family support is always important, but even more critical in women with established cardiac disease. This may not have been discussed before, as doctors tend, understandably, to avoid the issue of premature death.
Pre-pregnancy counselling opportunities are not limited to dedicated referrals, but should form part of the outpatient review. Reliance on undertaking pre-pregnancy counselling on demand should be avoided, as approximately 30% of pregnancies in the UK are unplanned. As such, cardiologists should be proactive in discussing the issues a pregnancy may present in any woman of childbearing age who has a cardiac condition. It should be remembered that pregnancy can (and sometimes does) occur soon after puberty, and, thus, the ages of 12–14 years are probably the best to start discussing the issues.Where there is no local expertise, or a cardiologist feels uncomfortable discussing contraception and pregnancy issues, women should be referred on to an appropriate expert.
Pre-pregnancy assessment
When evaluating any woman to assess the risk of a future pregnancy, access to old notes is important, including any previous operation note. A major UK court judgement in 2009 confirmed that the absence of previous notes (which are subsequently shown to still be in existence) when advising about management is medico-legally indefensible if there is an adverse outcome as a result. Additional risk factors such as previous arrhythmia, heart failure or thromboembolic event should be sought, along with determining the need for anticoagulation. A full examination and functional assessment should take place:
- functional class (New York Heart Association [NYHA] classes I and II generally predict a good outcome)
- resting oxygen saturations
- chest X-ray
- 12-lead electrocardiogram (ECG)
- echocardiography
- exercise testing with oxygen saturation monitoring
- B-type naturietic protein (BNP)/pro-BNP assessment
- cardiac magnetic resonance imaging (CMRI)/angiography as indicated.
With exercise testing, oxygen saturations should be maintained with a good blood pressure response. As regards workload, achieving seven metabolic equivalents (METs) is empirically used to predict a good outcome in some institutions. An abnormal chronotropic response during exercise testing has been shown to correlate with abnormal maternal and foetal outcomes in women with congenital heart disease.2
BNP levels rise in normal pregnancy starting in the first trimester and are approximately double those of non-pregnant controls.3 BNP levels are higher in women with cardiac disease, particularly in women with left ventricular impairment. In those at risk of decompensation, a baseline BNP and serial assessment during pregnancy can be useful. A BNP ≤100 pg/ml during pregnancy had 100% negative predictive value for identifying a cardiac event in women with heart disease.4 It should be emphasised that BNP also rises in cases of severe pre-eclampsia.5
Medication
The pre-pregnancy review is a good opportunity to review medication and make any necessary changes prior to conception or define planned changes in early pregnancy, e.g. anticoagulation strategies. Women should be informed of the need to start folic acid prior to conception (current recommendation 400 µg when trying to conceive until three months of pregnancy) to reduce the risk of a neural tube defect. Cardiac drugs that are teratogenic/foetotoxic include:
- ACE inhibitors and angiotensin-receptor blockers
- spironolactone
- amiodarone
- statins
- eplerenone
- warfarin.
Foetal risk
Maternal heart disease is associated with an increased risk of foetal growth restriction and pre-term delivery (the latter is largely iatrogenic).6 Adverse neonatal outcome occurs in 20–28% of pregnancies in women with heart disease.7 The strongest predictors are:8,9
- maternal cyanosis
- left heart obstruction
- oral anticoagulants
- mechanical heart valve replacement
- maternal smoking
- multiple gestation.
Foetal growth restriction is an important cause of peri-natal morbidity and mortality, and may have late sequelae for the infant. The increased risk of small for gestational age babies and pre-term delivery has implications, both for the prospective parents, but also for neonatal services. In addition to the adverse affects of maternal cyanosis or impaired cardiac output, the foetus may be at risk through exposure to maternal medication. If the mother has congenital heart disease without a chromosomal abnormality, the recurrence rate in the offspring is approximately 5% (it does vary somewhat with the precise nature of the condition).10 Where there is a chromosomal abnormality, this rate may increase as high as 50%, e.g. in cases of 22q deletion. In autosomal dominant conditions such as Marfan syndrome, again 50% of any offspring will be affected.
Individual cardiac conditions
Ischaemic heart disease
Pregnancy increases the risk of acute myocardial infarction three- to four-fold and the majority are anterior infarcts.11 Ischaemic heart disease (IHD) was the most common cause of cardiac death in the last Confidential Enquiry into Maternal and Child Health (CEMACH) report.1 All women who died from acute myocardial infarction had identifiable risk factors, and, the majority of women who died from IHD, died post-partum. The median maternal age at death was 36 years. In part, this increase in IHD deaths reflects lifestyle factors, such as the prevalence of smoking in young women, obesity and delaying childbirth. Although acute myocardial infarction in pregnancy is usually secondary to atherosclerotic coronary artery disease, coronary artery dissection and acute coronary thrombosis are important causes. Women with IHD, but who are asymptomatic, have no inducible ischaemia on functional testing and good left ventricular function generally, do well in pregnancy. Those with inducible ischaemia should be revascularised prior to pregnancy. Women with left ventricular impairment, as a result of ischaemic damage, should be risk stratified according to the degree of ventricular impairment.
Aortopathies
Normal pregnancy is associated with mild aortic dilation and disruption of normal vessel architecture, and pregnancy is a risk factor for dissection in the absence of any underlying aortic abnormally. Women with pre-existing aortopathies, e.g. Marfan syndrome or bicuspid aortic valve, are at increased risk, although the association between aortic dissection during pregnancy in such women may not be as strong as previously thought.12-14 When attempting to risk stratify women it is important to think beyond the absolute root dimension. The risk of dissection is multi-factorial. The risk of dissection depends on:
- the absolute dimension
- the rate of change
- the type of aortopathy
- family history of dissection.
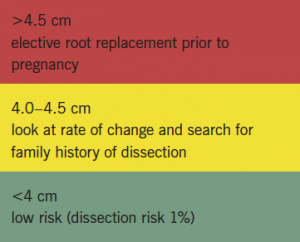
Women with a root >4.5 cm should be counselled about an elective root replacement prior to becoming pregnant (box 1). Women where the root is changing, but yet to reach this cut-off, should be counselled to wait while serial measurements are made. It should be remembered that women with Turner syndrome are often short statured and should have their aortic dimension referenced according to their body surface area. Women with Marfan syndrome taking losartan should be aware of the need to discontinue this once they have a positive pregnancy test. Women with aortopathy are treated with beta blockers throughout pregnancy, although data for this are not strong.
Turner syndrome
Recombinant human growth hormone and the availability of assisted reproduction have dramatically impacted the lives of women with Turner syndrome. However, these women are at increased risk of cardiovascular complications in later life, and pregnancy is associated with an adverse maternal and foetal outcome. Turner syndrome is associated with hypertension, an aortopathy, aortic coarctation, and, in addition, an increased risk of aortic dissection (up to 100-fold in young and middle-aged women).15 When assessing aortic size, it should be remembered that many of these women are short statured and a Z-score >2 or an aortic root diameter >2.5 cm/m2 is significantly dilated. Pregnancy-induced hypertension and pre-eclampsia are common. In one study, pre-term birth occurred in 38% of pregnancies. It correlated with a pregnancy-associated hypertensive disorder, and almost one in three babies were growth restricted. Only 40% of pregnancies had both a normal maternal and foetal outcome.16 The American Society for Reproductive Medicine guidelines requires cardiovascular screening of women with Turner syndrome prior to oocyte donation. As a minimum, women with Turner syndrome should have:
- 24-hour blood pressure
- transthoracic echo
- MRI of whole aorta (often tortuous and dilated).
A pregnancy in a woman with Turner syndrome requires careful multi-disciplinary management.
Peri-partum cardiomyopathy

Peri-partum cardiomyopathy (PPCM) is a non-familial, non-genetic form of dilated cardiomyopathy associated with pregnancy. It is defined as an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy, or in the months following delivery, where no cause of heart failure is found.17 The incidence of PPCM varies geographically and is ethnicity related, affecting approximately one in 3,000 pregnancies in Western Europe and the USA, rising to one in 1,000 in South Africa and one in 300 in Haiti. Risk factors include advanced maternal age, hypertension, multi-parity and black ethnicity (table 1).
PPCM is an inflammatory condition with high levels of oxidative stress, impaired microvascular function and apoptosis. However, the exact cause of PPCM remains unknown. Experimental data and early single-centre data suggest oxidative stress and cleavage of the hormone prolactin into an anti-angiogenic, pro-apoptotic subfragment may initiate and drive PPCM.18,19 There is interest that this same process may be involved in pre-eclampsia, a condition also of endothelial dysfunction. Although PPCM is typically termed a non-genetic, non-familial condition, a recent paper from the Netherlands found that a subset of women with a diagnosis of PPCM had a previously undiagnosed familial dilated cardiomyopathy (DCM), which raises the issue of screening family members of patients with PPCM who do not recover function post-partum.20
The outcome from PPCM is superior when at diagnosis:
- the left ventricular end diastolic dimension is <5.5 cm
- ejection fraction is >30%
- the cardiac troponin value is low.
A troponin value within two weeks of diagnosis has been found to correlate with the ejection fraction at six months.21
All women with a previous PPCM should receive pre-pregnancy counselling, as one of the most important issues is the risk of recurrence in a future pregnancy. The literature is small, is solely derived from USA populations and some is 10 years old, but there are common themes. In the much quoted study by Elkayam et al.,22 which is now over a decade old, women who had normalised their left ventricles (defined as ejection fraction of at least 50%) had a 21% chance of developing symptoms of heart failure, and 21% had a reduction in left ventricular function (ejection fraction) of more than 20%. However, none of the women died in a subsequent pregnancy. These values contrast with the cohort of women who embarked on a further pregnancy without normalising their left ventricular function. In this group, 44% developed symptoms of heart failure, 25% had a decrease in their left ventricular function of greater than 20% and 19% died. Furthermore, there was a greater incidence of women failing to normalise their ventricular function after the second pregnancy (14% of women in the first group compared with 31% in the latter had impaired systolic function at the last follow-up).
Women should be advised against a further pregnancy when there is:
- poor systolic function at diagnosis (ejection fraction ≤25%)
- failure to recover left ventricular function at six months from diagnosis.
Even women who normalise their ventricles should be aware that they risk deterioration in left ventricular function in a future pregnancy, which may not recover.
There are limited data for the role of exercise echo in assessing contractile reserve in women who appear to have normalised their left ventricular function, prior to embarking on another pregnancy.23
Non-PPCM
PPCM is not the only myocardial disease to complicate pregnancy. In a recent study of over 14 million hospital admissions for pregnancy in the USA, the rate of hospitalisation for cardiomyopathy was 0.46 per 1,000 deliveries. Of these, 0.18 were for PPCM and 0.28 for other cardiomyopathies.24 Pregnancy negatively impacts on an abnormal ventricle, whatever the aetiology, and women with known DCM need careful counselling about cardiac decompensation during pregnancy and the potential for left ventricular function not to recover post-partum back to the pre-pregnancy baseline.
The impact of pregnancy was assessed in a Canadian study of women with DCM due to prior doxorubicin exposure or idiopathic DCM.25 Women with PPCM were excluded. Non-pregnant women with DCM, and a similar degree of impairment, acted as controls. The investigators found that moderate or severe left ventricular impairment (ejection fraction ≤44%) and being in NYHA class III or IV were the main determinants of an adverse maternal cardiac event, defined as pulmonary oedema, cerebrovascular accident, arrhythmia requiring treatment, angina, myocardial infarction, cardiac arrest or cardiac death. There was a trend towards a pre-pregnancy history of an adverse cardiac event as being predictive.
Interestingly, adverse foetal events, defined as pre-term delivery, small for gestational age, respiratory distress syndrome, cerebral intraventricular haemorrhage and death, also occurred in women with mild left ventricular impairment (ejection fraction 45–54%) and in women in NYHA classes I and II, although most neonatal complications occurred in women with moderate or severe left ventricular impairment and/or NYHA class III/IV. Adverse obstetric factors, e.g. advanced maternal age and smoking, were also important. Pregnancy had a negative impact on the clinical course of women with DCM over a 16-month period, compared with non-pregnant DCM controls (although there is a difference between medication in the two groups since non-pregnant controls would have been on ACE inhibitors compared with their pregnant counterparts). There were no maternal deaths in the study but maternal morbidity was high.
Hypertrophic cardiomyopathy
The majority of women with hypertrophic cardiomyopathy tolerate pregnancy well. A minority of women report symptoms, usually breathlessness, chest pain and palpitations. The majority of these women were symptomatic prior to pregnancy.26 Pulmonary oedema can occur but is unusual and maternal mortality is rare. The presence of significant left ventricular outflow tract obstruction does not appear to influence maternal outcome (although care must be taken with epidural analgesia). Beta blockers should be considered in women with a history of arrhythmia and/or symptomatic or significant outflow tract obstruction.
Obstructive left heart conditions
Aortic stenosis
Women with mild aortic stenosis are low risk. Women with moderate or severe stenosis need rigorous assessment prior to pregnancy. Whereas once severe stenosis was an absolute contraindication to pregnancy, recent data have been more reassuring; although 10% of women with severe aortic stenosis (not risk stratified as below) experienced cardiac complications in pregnancy and 41% required surgery during follow-up.27 Symptomatic women, and those who have impaired left ventricular function and/or an abnormal exercise response on formal testing, should have intervention to their valve prior to pregnancy. Women with moderate or severe stenosis are deemed low risk if they fulfil the following criteria:
- normal 12-lead ECG
- normal left ventricular function on echo
- normal exercise capacity without ST change and a normal blood pressure response on formal exercise testing.
Women with moderate or severe aortic stenosis who have undergone pregnancy appear to be at increased risk of late cardiac events compared with non-pregnant women, and this should be raised during pre-pregnancy counselling.28
Mitral stenosis
Moderate or severe mitral stenosis is poorly tolerated due to the volume loading and tachycardia of pregnancy. The valve lesion is associated with significant morbidity. Women with anything other than mild mitral stenosis should be advised to delay pregnancy until the valve is intervened on, ideally with balloon valvuloplasty where appropriate. Women with mild mitral stenosis may become symptomatic, but generally tolerate a pregnancy well. Where there is doubt, exercise testing should be performed pre-pregnancy. Exercise echo, to assess the rise in transmitral gradient and alteration in pulmonary pressures, may also be useful.
Mitral and aortic regurgitation
In general, regurgitant lesions are well tolerated in pregnancy due to the drop in systemic vascular resistance, providing women have good left ventricular function, normal pulmonary pressures and a normal exercise capacity. Pre-pregnancy counselling in women with anything more than mild regurgitation and normal left ventricular function and dimensions, should include formal exercise testing. Where there is borderline left ventricular function, exercise echocardiography can be useful, although data to underpin this approach are lacking. Women with severe regurgitation, who are symptomatic and/or have left ventricular impairment, should be intervened on prior to pregnancy as they are at high risk of developing heart failure.29 Some women with aortic regurgitation will have concomitant aortic root dilation, and this should be carefully assessed including any recent change in dimension to suggest instability of the aorta.
Prosthetic valve replacements
There is no ideal heart valve replacement for a woman of childbearing age. The freedom from structural deterioration afforded by a mechanical prosthesis is offset by the need for anticoagulation with warfarin, which is teratogenic in higher doses and carries the risk of increased foetal loss and foetal intracranial haemorrhage. Although a bio-prosthesis will not require formal anticoagulation, it has a short valve lifespan in younger patients and is associated with structural deterioration. Approximately 50% of women of childbearing age will require re-operation 10 years after their original surgery.30 All women of childbearing age requiring valve surgery should have careful counselling about the type of valve replacement. Consideration of a homograft or a Ross procedure, which has good long-term outcome data,31 should be made where appropriate.
Women with normally functioning bio-prostheses and good left ventricular function usually tolerate pregnancy well. Those with stenotic bio-prostheses should be managed and risk stratified in the same way as those with native stenotic valve disease. I think the most difficult group of women to manage during pregnancy are women with mechanical valve replacements, and there are no controlled trials to guide optimal management. In the Dutch ZAHARA I study,9 the presence of a mechanical valve replacement was the strongest predictor of maternal complications. All women intending a pregnancy must be seen for pre-pregnancy counselling so a management plan can be agreed in the event of a positive pregnancy test, and so they are made aware of the maternal and foetal risks, and the fact that any pregnancy will require intense multi-disciplinary care.
Warfarin throughout pregnancy offers the best protection against maternal thrombosis, which may be fatal to the mother and foetus. In a review of the literature, the maternal thromboembolism risk with oral anticoagulants was 3.9% with a maternal haemorrhage risk of 2.5%, mostly occurring peri-delivery.32 However, warfarin crosses the placenta and is associated with a 6–10% embryopathy risk, although the risk can be minimised when the maternal warfarin dose is ≤5 mg,33 or if heparin is substituted by week 6 of pregnancy and continued until week 12. Warfarin is also associated with an appreciable risk of foetal loss, foetal malformation and pre-term delivery (up to 36% of women deliver before 37 weeks).
Unlike warfarin, heparin does not cross the placenta, but is associated with a higher risk of maternal valve thrombosis and thromboembolism, and is associated with osteoporosis and maternal thrombocytopenia. Thromboembolism was higher with unfractionated heparin regimens, which have been superseded by twice-daily, weight-adjusted, low molecular weight heparin (LMWH) regimens with regular anti-Xa level monitoring. A maternal thrombosis rate of 10.6% was reported in one study from New Zealand, but in all cases compliance was suboptimal.34 Heparin use also has better foetal outcomes. In the New Zealand study, 96% of women, taking predominately LMWH (enoxaparin), had a surviving infant compared with 75% of women taking primarily warfarin.34
There is no single accepted anticoagulation strategy for metallic valve replacements and an individualised strategy should take into account the patient’s wishes and past medical history. The anticoagulant options are:
- warfarin throughout – preferred when the dose is <5 mg daily, in women with a history of thromboembolism or in those with a ball and cage valve replacement in the mitral position
- LMWH started prior to week 6 gestation and continued to week 12, warfarin to week 36 and then switch to LMWH pre-delivery
- LMWH throughout.
The newer anticoagulant agents are not licensed in pregnancy, nor are they licensed for use with prosthetic valves.
Congenital heart disease
The number of adults with congenital heart disease now outnumbers children, reflecting improved surgical outcomes and medical care over recent decades. Although the number of women with congenital heart disease who die in pregnancy is small, it remains a considerable cause of maternal and foetal morbidity. Pre-pregnancy counselling should be undertaken by those with expertise in congenital heart disease. In broad terms, women who saturate normally, in a good functional class with normal left ventricular function, do well in pregnancy. Disease specific risk can be assessed using scoring systems, e.g. CARPREG score8 (acquired and congenital heart disease) and the predictors from the ZAHARA study.9 These scoring systems are highly dependent on the population studied. Important determinants of risk, such as aortopathy and pulmonary hypertension, were not included due to underrepresentation of these conditions in the studied populations. Furthermore, valid data on ventricular function were not available in the ZAHARA study and, hence, impaired function could not be identified as a risk factor in that cohort. The European Society of Cardiology Task Force on the management of cardiovascular disease in pregnancy recommends use of the modified World Health Organization (WHO) risk classification (box 2).7

cardiovascular risk(7)
Pulmonary hypertension
Pulmonary hypertension (PHT), defined at cardiac catheter as a mean pulmonary pressure ≥25 mmHg at rest or 30 mmHg on exercise, is a challenging, progressive disease. In older series, a maternal mortality of 30–50% was reported, with deaths typically occurring in the third trimester and in the first weeks post-partum due to pulmonary hypertensive crises and right heart failure. Recent advances in the medical management of PHT have led to improvements in symptoms and survival, and more recent studies have reported better maternal outcomes for women with PHT, with maternal mortality at 17–33%.35-37 While this is an improvement, the prospect of death is high, and some targeted therapy drugs, e.g. bosentan and ambrisentan, are teratogenic. Careful counselling in a PHT centre is mandatory.
Conclusion
All cardiologists should recognise the need to raise the issue of pregnancy whenever a diagnosis of acquired heart disease is made in a woman of childbearing age, and refer on as their expertise dictates. Pre-pregnancy counselling should be viewed as the mainstay of clinical practice and, in the case of women with congenital heart disease, it should start early during transition. Although women with heart disease are at increased risk during pregnancy, the majority of women have a good outcome.
It should be emphasised that, in the rare circumstances when a woman has been recommended to avoid or terminate a pregnancy, if she decides to proceed with the pregnancy, she should be reassured that she will continue to be supported and cared for.
Conflict of interest
None declared.
Key messages
- Cardiac disease remains the commonest cause of maternal death in the UK
- Pre-pregnancy counselling for women with congenital or acquired heart disease should form part of the outpatient review in women of childbearing age
- Where there is no local expertise women should be referred on
References
1. Wilkinson G. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer – 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118(suppl 1):1–205. http://dx.doi.org/10.1111/j.1471-0528.2010.02847.x
2. Lui GK, Silversides CK, Khairy P et al. Heart rate response during exercise and pregnancy outcome in women with congenital heart disease. Circulation 2011;123:242–8. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.953380
3. Hameed AB, Chan K, Ghamsary M et al. Longitudinal changes in the B-type natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol 2009;32:E60–E62. http://dx.doi.org/10.1002/clc.20391
4. Tanous D, Siu SC, Mason J et al. B-type naturietic peptide in pregnant women with heart disease. J Am Coll Cardiol 2010;56:1247–53. http://dx.doi.org/10.1016/j.jacc.2010.02.076
5. Resnik JL, Hong C, Resnik R et al. Evaluation of B-type naturietic peptide (BNP) in normal and preeclamptic women. Am J Obstet Gynecol 2005;193:450–4. http://dx.doi.org/10.1016/j.ajog.2004.12.006
6. Gelson E, Curry R, Gatzoulis MA et al. Effect of maternal heart disease on fetal growth. Obstet Gynecol 2011;117:886–91. http://dx.doi.org/10.1097/AOG.0b013e31820cab69
7. Regitz-Zagrosek V, Blomsrom Lundqvist C, Borghi C et al. ESC guidelines on the management of cardiovascular disease during pregnancy: the Task Force on the Management of Cardiovascular Disease during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–97. http://dx.doi.org/10.1093/eurheartj/ehr218
8. Siu SC, Sermer M, Colman JM et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001;104:515–21. http://dx.doi.org/10.1161/hc3001.093437
9. Drenthen W, Boersma E, Balci A et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31:2124–32. http://dx.doi.org/10.1093/eurheartj/ehq200
10. Burn J, Brennan P, Little J et al. Recurrence risks in the offspring of adults with major heart defects: results from first cohort of British collaborative study. Lancet 1998;351:311–16. http://dx.doi.org/10.1016/S0140-6736(97)06486-6
11. Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol 2008;52:171–80. http://dx.doi.org/10.1016/j.jacc.2008.03.049
12. McKellar SH, MacDonald RJ, Michelena HI, Connolly HM, Saundt TM. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol 2011;107:96–9. http://dx.doi.org/10.1016/j.amjcard.2010.08.061
13. Drenthen W, Pieper PG, Roos-Hesselink JW et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol 2007;49:2303–11. http://dx.doi.org/10.1016/j.jacc.2007.03.027
14. Thalmann M, Sodeck GH, Domanovits H et al. Acute type A aortic dissection and pregnancy: a population-based study. Eur J Cardiothorac Surg 2011;39:e159–e163. http://dx.doi.org/10.1016/j.ejcts.2010.12.070
15. Bondy C. Aortic dissection in Turner syndrome. Curr Opin Cardiol 2008;23:519–26. http://dx.doi.org/10.1097/HCO.0b013e3283129b89
16. Chevalier N, Ltur H, Lelannou D et al. Materno-fetal cardiovascular complications in Turner syndrome after oocyte donation: insufficient prepregnancy screening and pregnancy follow-up are associated with poor outcome. J Clin Endocrinol Metab 2011;96:E260–E267. http://dx.doi.org/10.1210/jc.2010-0925
17. Sliwa K, Hilfiker-Kleiner D, Petrie M et al. Current state of knowledge on aetiology, diagnosis, management and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Failure 2010;12:767–78. http://dx.doi.org/10.1093/eurjhf/hfq120
18. Hilfiker-Kleiner D, Kaminski K, Podewski E et al. A cathepsin D-cleaved 16kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. http://dx.doi.org/10.1016/j.cell.2006.12.036
19. Sliwa K, Blauwet L, Tibazarwa K et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 2010;121:1465–73. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.901496
20. Van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 2010;121:2169–75. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.929646
21. Hu CL, Li YB, Zou YG et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart 2007;93:488–90. http://dx.doi.org/hrt.2006.087387v1
22. Elkayam U, Tummala PP, Rao K et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 2001;344:1567–71. http://dx.doi.org/10.1056/NEJM200105243442101
23. Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Obstet Gynecol 2010;109:34–6. http://dx.doi.org/10.1016/j.ijgo.2009.10.011
24. Kuklina EV, Callaghan WM. Cardiomyopathy and other myocardial disorders among hospitalizations for pregnancy in the United States: 2004-2006. Obstet Gynecol 2010;115:93–100. http://dx.doi.org/10.1097/AOG.0b013e3181c4ee8c
25. Grewal J, Siu SC, Ross HJ et al. Pregnancy outcomes in women with dilated cardiomyopathy. J Am Coll Cardiol 2009;55:45–52. http://dx.doi.org/10.1016/j.jacc.2009.08.036
26. Thaman R, Varnanva A, Hamid MS et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart 2003;89:752–6. http://dx.doi.org/10.1136/heart.89.7.752
27. Silversides C, Colman J, Sermer M, Farine D, Siu SC. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol 2003;91:1386–9. http://dx.doi.org/10.1016/S0002-9149(03)00340-0
28. Tzemos N, Silversides CK, Colman JM et al. Late cardiac outcomes after pregnancy in women with congenital aortic stenosis. Am Heart J 2009;157:474–80. http://dx.doi.org/10.1016/j.ahj.2008.10.020
29. Lesniak-Sobelga A, Tracz W, KostKiewicz M, Podolec P, Pasowicz M. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases – maternal and fetal outcome. Int J Cardiol 2004;94:15–23. http://dx.doi.org/10.1016/j.ijcard.2003.03.017
30. Elkyam U, Bitar F. Valvular heart disease and pregnancy: part II. Prosthetic valves. J Am Coll Cardiol 2005;46:403–10. http://dx.doi.org/10.1016/j.jacc.2005.02.087
31. De Kerchove L, Rubay J, Pasquet A et al. Ross operation in the adult: long-term outcomes after root replacement and inclusion techniques. Ann Thorac Surg 2009;87:95–102. http://dx.doi.org/10.1016/j.athoracsur.2008.09.031
32. Chan WS, Ananad S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000;160:191–6. http://dx.doi.org/10.1001/archinte.160.2.191
33. Vitale N, De Feo M, Salvatore L et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol 1999;33:1637–41. http://dx.doi.org/10.1016/S0735-1097(99)00044-3
34. McLintock C, McCowan LME, North RA. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG 2009;116:1585–92. http://dx.doi.org/10.1111/j.1471-0528.2009.02299.x
35. Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009;30:256–65. http://dx.doi.org/10.1093/eurheartj/ehn597
36. Kiely DG, Condliffe R, Webster V et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG 2010;117:565–74. http://dx.doi.org/10.1111/j.1471-0528.2009.02492.x
37. Duarte AG, Thomas S, Safdar Z et al. Management of pulmonary arterial hypertension during pregnancy. A retrospective, multicenter experience. Chest 2013;143:1330–6. http://dx.doi.org/10.1378/chest.12-0528
