Contrast-induced nephropathy is the third most common cause of in-hospital acute kidney injury and accounts for 10% of total cases. It is commonly encountered following coronary angiography and this systematic review aims to use current evidence to ascertain which treatment modalities are most effective in the prevention of the disease.
A PubMed literature search was conducted in March 2014 using search terms, ‘contrast nephropathy and coronary angiography’. The data analysed included 15 trials and two meta-analyses in order to determine whether patients given N-acetylcysteine (NAC), sodium chloride or sodium bicarbonate had better clinical outcomes. Study data were reviewed and quality of data discussed.
Current data indicate that sodium bicarbonate is as effective as sodium chloride when used in patients with estimated glomerular filtration rate (eGFR) <60 ml/min. NAC adds no statistically significant benefit in mild-to-moderate renal disease regardless of whether it is used in isolation or as an adjunct therapy with fluid.
Pathogenesis
With the advent of the iodinated contrast study came the complication of contrast-induced nephropathy (CIN), an increasingly recognised and discussed iatrogenic disease process seen with coronary angiography. As we become more invasive in our cardiac investigations, and coronary interventions become more widespread, coronary angiography is offered to more and more patients. Therefore, a better understanding of the treatment modalities aimed at minimising the risk of CIN has never been more important.
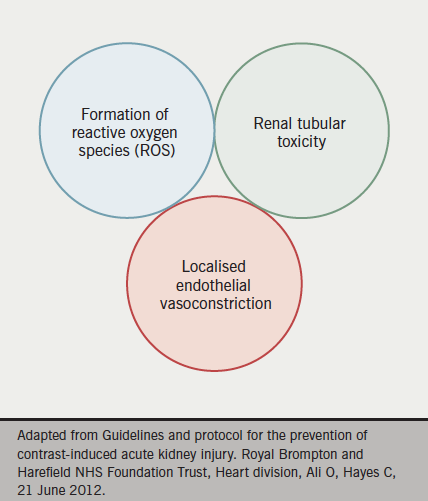
CIN is a direct hyper-acute tubular insult on the filtering mechanisms of the kidney. Although still poorly understood, it is postulated that free radical accumulation and subsequent damage to epithelial layers, as well as compromised renal perfusion, are important (see figure 1). CIN is now the third most common cause of in-hospital acute kidney injury and is responsible for approximately 10% of all cases of iatrogenic renal disease.1 In the short term, it is associated with a transient rise in serum creatinine levels, however, this can progress in a proportion of cases to severe acute kidney injury, metabolic acidosis, hyperkalaemia or anuria requiring haemofiltration or haemodialysis.
Joint guidance set out in April 2013 by the British Cardiovascular Intervention Society, Royal College of Radiologists and Renal Association redefined the biochemical criteria of CIN as a rise of serum creatinine levels post-intravenous contrast of more than 1.5 times baseline within seven days, an absolute increase of more than 26 µmol/L within two days, or a urine output of less than 0.5 ml/kg/hr for more than six hours post-procedure.2 Prior to this guidance, the generally accepted definition of CIN was a rise of serum creatinine above baseline of more than 25%, or an absolute increase of at least 44 µmol/L. The data provided in this article uses this definition unless otherwise stipulated, since there have been limited data collected since the re-classification of CIN.
Clinical significance
In 2012, Gallagher et al. explored the long-term outcomes of patients who underwent primary percutaneous coronary intervention (PPCI) for ST-segment elevation myocardial infarction (STEMI) and, retrospectively over seven years, it was observed that within a standardised cohort of 2,224 patients, CIN was associated with both increased short- and long-term mortality.3 As well as recognising that older patients are at increased risk of CIN (mean age of 69.3 vs. 63.7 years, p<0.0001), it was observed that females, as opposed to males, incur poorer outcomes (30.0% vs. 22.6%, respectively, p=0.004). Furthermore, Gallagher et al. found that those with baseline renal disease, i.e. an estimated glomerular filtration rate (eGFR) of less than 60 ml/min, experienced a statistically significant increased risk of CIN post-contrast (33.1% vs. 18.6%, respectively, p<0.0001). Taken together with other comorbidities, such as diabetes mellitus, hypertension, cholesterol status and reduced left ventricular ejection fraction, it was realised that CIN can be a predictor of significant all-cause mortality within 30 days (16.4% vs. 2.0%, p<0.0001) and at three years (22.4% vs. 5%, p<0.0001).
An earlier retrospective study of 3,236 patients, by From et al.,4 supports the results of Gallagher et al.’s work.3 They reported a statistically significant increase in all-cause mortality in an 809 patient cohort who developed CIN at 30 days with an odds ratio of 3.37 (95% confidence interval [CI] 2.58–4.41, p<0.001) as opposed to the 2,427 patients who did not develop CIN.4
Rudnick and Feldman conducted their own review of five observational studies and also concluded that acute kidney injury associated with the administration of contrast is, at the very least, a marker of increased mortality and prolonged hospital stay. They also concluded that peri-procedural events associated with CIN were compounded by other comorbidities mentioned previously, such as pre-existing coronary artery disease and diabetes mellitus. Furthermore, it was found that cumulative mortality can be extended to as much as one year post-contrast, as death rates among those with CIN was 22.6% with pre-existing chronic renal dysfunction when compared with 6.9% without (p<0.0001).5
The overwhelming conclusion from these studies is of an increase in both short- and long-term mortality as a consequence of CIN, as well as increased length of hospitalisation. Although the data are largely retrospective, the observational studies do suggest a tangible association between CIN and mortality, which highlights the importance of early recognition and effective treatment of CIN in those undergoing coronary angiography ± percutaneous coronary intervention (PCI).
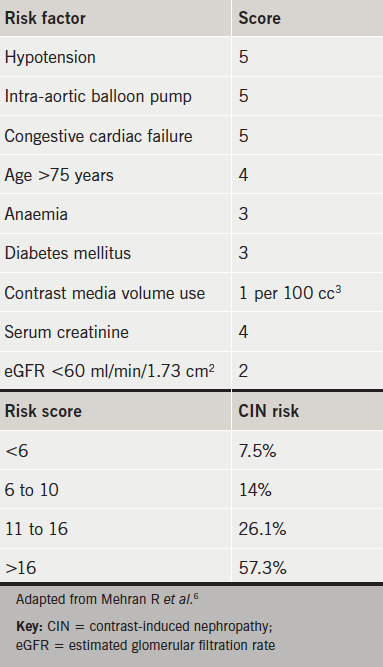
The Mehran scoring system was adopted specifically for CIN risk stratification in patients undergoing PCI and has been validated in an 8,357 strong patient cohort, which included ‘high-risk’ patients.6 Although not universally accredited by specialist colleges, it has been validated in groups undergoing both elective and emergency procedures for STEMI and non-STEMI.7,8 Early recognition of those who may require renal replacement therapy should be sought using this tool, and less invasive functional studies should be considered if at high risk (see table 1). However, when coronary angiography and/or PCI is the most appropriate intervention, the CIN prevention strategies discussed below should be explored.
Treatment strategies to reduce the risk of CIN
N-acetylcysteine
The most widely utilised prophylaxis and treatment strategies for CIN in coronary interventions are intravenous fluids and oral N-acetylcysteine (NAC). Although the precise pharmacokinetics of NAC remain somewhat of a mystery, it may play a role in the consumption of reactive oxygen species and the liberation of both nitric oxide and N-nitrosothiol, which in turn are known to cause endothelial vasodilation.9
Early studies by Tepel et al. investigated the use of 600 mg twice-daily oral NAC as prophylaxis against CIN in computed tomography (CT) scans. This study found that the mean serum creatinine improved from 2.5 mg/dL to 2.1 mg/dL (±1.3 mg/dL, p<0.001) when compared with control, and, given these promising results, the practice was extrapolated to cardiac catheterisation.10 However, an extensive meta-analysis in 2006 by the same author, citing 26 prospective randomised trials, 25 being specific for CIN in coronary angiography, failed to show any significant relative risk reduction with NAC versus placebo.11 Another study by Briguori’s team reported a relative risk of 0.3 (p=0.02) with NAC at 1200 mg twice daily, while others reported little if any improvement in serum creatinine levels with lower dosage regimens.12
Kshirsagar et al. also conducted a meta-analysis of 16 prospective trials including 1,538 patients.13 In contrast to the work conducted by Tepel, CIN was assessed in patients undergoing a broad range of contrast studies, not limited to coronary angiography. The result was heterogeneic data, and clinically significant conclusions regarding the effect of NAC could not be drawn. Despite some patient groups receiving high-dose NAC and others low dose, Kshirsagar found no statistical difference in CIN incidence. However, it was recognised that elderly patients and those with diabetes mellitus are at increased risk of contrast-induced kidney injury.
Discrepancies in the data analysing the effect of NAC have been attributed to variances in the bioavailability of glutathione (the active metabolite of NAC), differences in therapeutic dosages, variances of contrast media concentration and differences in dose timing between the various studies. A common theme throughout the studies is the heterogeneity of the data and, despite the fact that later research failed to replicate the positive effect of NAC observed in early studies with 600 mg twice-daily dosing, it continues to be used in many hospital trusts despite a lack of evidence base.
Sodium bicarbonate versus sodium chloride
Volume expansion using either sodium chloride or sodium bicarbonate facilitates the renal clearance of contrast media, with bicarbonate having the additional benefit of urinary alkalisation, thereby lowering free radical burden on the renal tubules. Merten et al. conducted a prospective randomised trial of 119 standardised cases with stable renal disease and a serum creatinine of at least 97.2 µmol/L.14 Subjects were given intravenous fluids in the form of either sodium chloride or sodium bicarbonate from one hour prior to the contrast study at 3 ml/kg/hr until six hours post-procedure at a rate of 1 ml/kg/hr. It was found that in those subjects who underwent cardiac catheterisation, the group who received sodium bicarbonate had a CIN incidence of 2.0%, whereas those administered sodium chloride had a greater incidence of CIN at 16.7%. The calculated mean difference in relative risk between the two arms was significant at 14.7% (95% CI 3.4–25.9%, p=0.02) with an improvement in eGFR in the sodium bicarbonate group of 8.6% (95% CI 0.2–17.2%, p=0.02) in comparison to those who were administered sodium chloride. This resulted in an absolute CIN risk reduction of 11.9% with the use of sodium bicarbonate.
Given the growing evidence base for the use of sodium bicarbonate as prophylaxis, Tamai et al. attempted to assess whether there is a dose-dependent effect on the incidence of CIN with different concentrations of the fluid.15 There were 123 patients randomised into a group given 833 mEq/L sodium bicarbonate and a further group given only 160 mEq/L. After two days, the incidence of CIN was significantly lower in those who were infused with the higher dose (0% vs. 17.3%, p=0.005) and subsequent percentage changes in serum creatinine levels were again markedly lower in the higher dose group (–2.65 ± 9.83% vs. 9.14 ± 14%, p=0.001).
Despite Merten and Tamai observing statistically significant results with sodium bicarbonate, it has become common practice to adjunct intravenous fluid simultaneously with 600 mg NAC prior to coronary angiography in those at risk of CIN. Prospective randomised studies by Brar et al. and Maioli et al., with a total patient cohort of 855, produced similar results when assessing CIN incidence in patients given either sodium bicarbonate or sodium chloride in conjunction with 600 mg NAC twice daily.16,17 Of these, Brar et al. enrolled 352 standardised subjects, all of whom had an eGFR of less than 60 ml/min. CIN occurred in 13.3% of patients receiving sodium bicarbonate and 14.6% of patients receiving sodium chloride with a relative risk of 0.94 (95% CI 0.55–1.60, p=0.82). It was observed that the relative risks of all-cause mortality, dialysis and cardiovascular events were similar between sodium chloride and sodium bicarbonate at 30 days and six months. Of those who developed significant nephropathy, six-month mortality was 9.5% in the sodium chloride group and 10.0% in the sodium bicarbonate group (p >0.99).
Maioli et al. prospectively randomised 502 patients, all of whom had an eGFR <60 ml/min prior to contrast study. They were randomised to receive NAC at 600 mg twice daily and either sodium chloride or sodium bicarbonate. The data suggest that both groups experienced a non-statistically different rise in baseline creatinine of 0.14 mg/dL (± 0.3) in the sodium chloride arm, and 0.15 mg/dL (± 0.4) in the sodium bicarbonate arm (p=0.78). Over the 502 patient case series, all-cause mortality was not significantly different between the two groups with an incidence of 1.2% with sodium chloride and 1.6% with sodium bicarbonate (p=0.99). However, only 15% of patients enlisted in Maioli’s study had chronic kidney disease (CKD) stage III or worse.
Thus, to investigate the rationale of high-dose NAC at 1,200 mg twice daily with intravenous fluid in more severe forms of renal disease, Briguori et al. enlisted 326 patients with an eGFR <40 ml/min in the Renal Insufficiency Following Contrast Media Administration(REMEDIAL) trial.18 Patients were randomised to 1,200 mg twice-daily NAC and either intravenous sodium chloride or sodium bicarbonate. Briguori’s team found a significant CIN incidence of 9.9% in those who received sodium chloride and NAC, but only 1.9% of patients developed CIN when administered NAC and sodium bicarbonate (p=0.019). It was concluded, therefore, that combined treatment of high-dose NAC with sodium bicarbonate can predict better outcomes in a very high-risk subset of patients with a statistically significant lower incidence of CIN.
The REMEDIAL data were replicated in the RENO study by Recio-Mayoral et al., who identified a possible benefit with sodium bicarbonate and even higher-dose NAC than used in previous studies in the prevention of CIN in those patients undergoing emergency PPCI.19 Unlike previous studies, patients were not excluded on the basis of eGFR alone, unless they were already on regular dialysis with end-stage renal failure. Sodium bicarbonate was administered, in line with the standard in Merten’s work, at 154 mEq/L for one hour pre-procedure, with the addition of 2,400 mg NAC intravenous loaded over one hour. Intravenous sodium bicarbonate was then given at a rate of 1.5 ml/kg/hr for six hours after the procedure, and a further two doses of 600 mg NAC were given orally the following day. Another group of patients received sodium chloride at 1 ml/kg/hr for 12 hours only after the procedure, and two 600 mg doses of NAC the next day. It was found that CIN developed in 1.8% of those who received the sodium bicarbonate protocol and in 21.8% of those given the sodium chloride protocol. Although, obviously, these were not randomised direct comparisons, they did find a statistically significant difference in CIN incidence of 20% (95% CI 8–31%, p=0.0009) by day 3 post-procedure. The REMEDIAL and RENO trials both highlight the potential dose-response variability of dual therapy (NAC and sodium bicarbonate). The data we have to date suggest that when high loading doses of NAC are paired with intravenous sodium bicarbonate, lower CIN rates are obtained in those patients with severe renal dysfunction. However, a more recent trial (ACT) found that NAC does not reduce the risk of CIN in high-risk patients undergoing coronary and peripheral angiography. In view of the conflicting data regarding the use of NAC, and lack of discernible and clinically relevant outcomes, the routine use of NAC should be reviewed and questioned.20
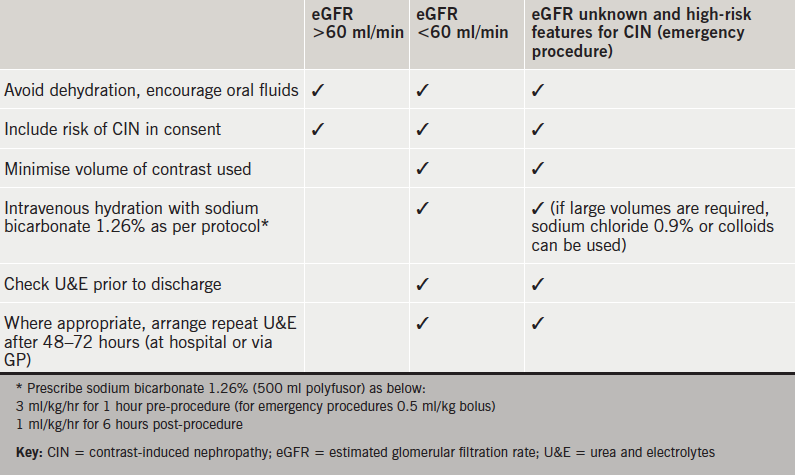
Based on current data and our local experience, we propose practical guidance to the prevention of CIN during coronary intervention (see table 2).
Conclusion
The current data suggest that the use of sodium bicarbonate is safe and as effective as sodium chloride in both low- and high-dose preparations, especially in high-risk patients with eGFR <60 ml/min. Furthermore, NAC, used both as monotherapy or in addition to sodium bicarbonate, has no clear benefit in those with mild stable renal disease.
Conflict of interest
None declared.
Key messages
- Contrast-induced nephropathy (CIN) is an increase in creatinine of more than 26 µmol/L within two days, 1.5 times baseline within seven days, or a urine output of less than 0.5 ml/kg/hr for more than six hours post-procedure
- CIN increases 30-day mortality by 14.4% and three-year mortality by 17.4%
- N-acetylcysteine (NAC) has conflicting data in the prevention of CIN post-coronary angiography
- Sodium bicarbonate can significantly reduce the incidence of CIN, especially in patients with moderate-to-severe renal failure
References
1. Gallagher S, Knight C. Contrast-induced nephropathy in primary percutaneous coronary intervention. Heart 2011;97:1723–5. http://dx.doi.org/10.1136/heartjnl-2011-300517
2. Lewington A, MacTier R, Hoefield R, Sutton A, Smith D, Downes M. Prevention of contrast induced acute kidney injury (CI-AKI) in adult patients. London: The Renal Association, The Royal College of Radiologists and The British Cardiovascular Intervention Society, 2013. Available from: https://www.rcr.ac.uk/publications.aspx?PageID=310&PublicationID=391
3. Gallagher S, Hassan S, Jones DA et al. Impact of contrast-induced nephropathy upon short and long-term outcomes of patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart 2012;98;A70. http://dx.doi.org/10.1136/heartjnl-2012-301877b.124
4. From A, Bartholmai B, Williams A et al. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc 2008;83:1095–100. http://dx.doi.org/10.4065/83.10.1095
5. Rudnick M, Feldman H. Contrast-induced nephropathy: what are the true clinical consequences? Clin J Am Soc Nephrol 2008;3:263–72. http://dx.doi.org/10.2215/CJN.03690907
6. Mehran R, Aymong, E, Nikolsky E et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:1393–9. http://dx.doi.org/10.1016/j.jacc.2004.06.068
7. Sgura F, Bertelli L, Monopoli D et al. Mehran contrast-induced nephropathy risk score predicts short and long term clinical outcomes in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv 2010;3:491–8. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.110.955310
8. Raingruber B, Kirkland-Walsh H, Chahon N, Kellerman M. Using the Mehran risk scoring tool to predict risk for contrast medium-induced nephropathy undergoing percutaneous angiography. Crit Care Nurse 2011;31:e17–e22. http://dx.doi.org/10.4037/ccn2011746
9. Fishbane S, Durham JH, Marzo K, Rudnick M. N-acetylcysteine in the prevention of radiocontrast-induced nephropathy. J Am Soc Nephrol 2004;15:251–60. http://dx.doi.org/10.1097/01.ASN.0000107562.68920.92
10. Tepel M, van der Giet M, Scwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180–4. http://dx.doi.org/10.1056/NEJM200007203430304
11. Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 2006;113:1799–806. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.595090
12. Briguori C, Colombo A, Airoldi F et al. N-acetylcysteine versus fenoldopam mesylate to prevent contrast agent-associated nephrotoxicity. J Am Coll Cardiol 2004;44:762–5. http://dx.doi.org/10.1016/j.jacc.2004.04.052
13. Kshirsagar AV, Poole C, Mottl A et al. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol 2004;15:761–9. http://dx.doi.org/10.1097/01.ASN.0000116241.47678.49
14. Merten GJ, Burgess W, Gray LV et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291:2328–34. http://dx.doi.org/10.1001/jama.291.19.2328
15. Tamai N, Ito S, Nakasuka K et al. Sodium bicarbonate for the prevention of contrast-induced nephropathy: the efficacy of high concentration solution. J Invasive Cardiol 2012;24:439–42. Available from: http://www.invasivecardiology.com/articles/sodium-bicarbonate-prevention-contrast-induced-nephropathy-efficacy-high-concentration-solu
16. Brar SS, Yuh-Jer Shen A, Jorgensen MB et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography. JAMA 2008;300:1038–46. http://dx.doi.org/10.1001/jama.300.9.1038
17. Maioli M, Toso A, Leoncini M et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 2008;52:599–604. http://dx.doi.org/10.1016/j.jacc.2008.05.026
18. Briguori C, Airoldi F, D’Andrea D et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 2007;115:1211–17. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.687152
19. Recio-Mayoral A, Chaparro M, Prado B et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO study. J Am Coll Cardiol 2007;49:1283–8. http://dx.doi.org/10.1016/j.jacc.2006.11.034
20. The ACT Trial Investigators. Rationale, design, and baseline characteristics of the acetylcystein for contrast-induced nephropathy (ACT) trial: a pragmatic randomized controlled trial to evaluate the efficacy of acetylcysteine for the prevention of contrast-induced nephropathy. Trials 2009;10:38. http://dx.doi.org/10.1186/1745-6215-10-38
