Current data show that PAH patients are now older than previously reported, and more patients are identified with associated co-morbidities, according to Professor Marius Hoeper, Hannover Medical School, Germany. One recent study in more than one thousand patients showed that over one half had two or more co-morbidities.1 About one third had systemic hypertension, 16% had hypothyroidism, 15% scleroderma, 14% were clinically depressed (possibly as a consequence of pulmonary hypertension) and a further 13% had diabetes.1 Some centres also report that the spectrum of PAH patients seems to be changing, he said. Whereas in the past idiopathic PAH was more common, in recent years PAH in association with connective tissue disorders and congenital heart disease has been more frequently observed in many centres.2
The severity of pulmonary vascular resistance at presentation also seems to be (slightly) declining, which suggests that the disease is being diagnosed earlier. However, the vast majority of patients are still in functional class III or IV at diagnosis.2 Professor Hoeper emphasised the improvements in available medical treatments, which have broadened from the limited options of intravenous epoprostenol and calcium channel blockers to include a wide range of prostanoids and the newer drug classes of endothelin receptor antagonists (three are currently available in some countries) and PDE-5 inhibitors.

It is very difficult to select PAH treatments, said Professor Hoeper, as there are only limited data to provide guidance. However, a number of factors are taken into consideration and these are shown in table 1.
Since many patients are being treated with combinations, the possibility of drug-drug interactions increases.3 Similarly, the mode and convenience of administration must be considered; for example many patients might prefer oral administration. Treatment costs and approval status are also considerations. There are disparities in the indications for these drugs across Europe, which is potentially a source of disagreement for several more years.
For prescribers, it is important to know that the drugs work and to see haemodynamic evidence of improvement. Looking at data from randomised controlled trials (RCTs), Professor Hoeper expressed the view that improvements in pulmonary artery pressure (PAP) are often very modest. Reviewing data from the SUPER-1 trial with sildenafil,4 some improvements in exercise capacity are observed but he argued that we do not yet know the most efficacious dose.
Endothelin receptor antagonists
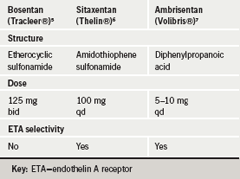
Professor Hoeper then described the characteristics of the three clinically developed endothelin receptor antagonists (ERAs), bosentan, sitaxentan and ambrisentan (table 2).5-7 These drugs have different chemical structures, with bosentan and sitaxentan belonging to the sulfonamide class and ambrisentan belonging to the propanoic acid class. Receptor affinities may confer differences in efficacy but presently this is difficult to determine. Six-minute walk distance data from placebo-controlled trials show very similar changes in most of the trials.8-10 Likewise, we do not yet have long-term survival data from RCTs, just the open-label extension data from several short-term RCTs. The survival observed at one year is in the high nineties: it appears to be very similar for the three ERAs, which makes choice of drug difficult.
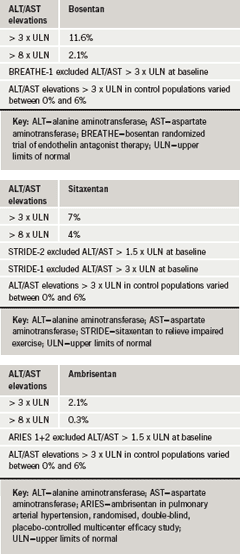
Factors such as safety also need to be taken into consideration. In Professor Hoeper’s view, rises in hepatic aminotransferases (e.g. alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) are a problem with these drugs in general and something that has to be monitored when on the drug continuously. The STRIDE 2 trial,9 sought to determine the optimal dose of the ERA, sitaxentan in PAH patients, and included an open-label bosentan arm for observation only. Although powered for efficacy, the study did report safety results as well, which showed that the incidence of elevated aminotransferases (>3 x upper limit of normal [ULN]) was 6% in the placebo group; 5% in the sitaxentan 50 mg group; 3% in the sitaxentan 100 mg group; and 11% for open-label bosentan. But in the long-term extension data,10 the difference seemed to be less pronounced (tables 3a-3c). For ambrisentan, the newest of the three ERAs, it appears that the incidence of aminotransferase elevation is lower than for the other two but this drug still has to stand the test of long-term administration, Professor Hoeper noted.-
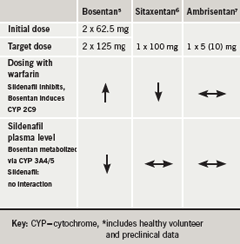
Another factor of concern, in his view, is drug-drug interaction, which is “becoming an issue in the treatment of pulmonary hypertension at the present time”. Bosentan and sitaxentan have the propensity to interact with warfarin (table 4):this is important because up to 75% of PAH patients are being treated with warfarin or other anticoagulants.11 With bosentan, the dose of warfarin has to be increased12 whereas with sitaxentan, the dose of warfarin has to be decreased in order to avoid bleeding problems.13 There is also an interaction between bosentan and sildenafil, which leads to lower concentrations of the latter.14 We don’t know if this has any clinical relevance, said Professor Hoeper, and this interaction is not seen with sitaxentan6 or ambrisentan.7
Summarising, he said that the majority of PAH patients in functional classes II/III are receiving oral medications such as PDE-5 inhibitors or ERAs. There are not sufficient data to compare their long-term efficacy, but there appears to be no difference in terms of efficacy among the three ERAs.Other factors such as side effects and costs must also be taken into consideration. Monotherapy, regardless of the agent used, is often not sufficiently effective in some of these patients, according to Professor Hoeper. Current clinical data from combination studies are limited but the results are promising. He presented findings from the REVEAL Registry,7 a multicentre, observational US-based study, looking at PAH treatment. It showed (figure 1) from a sample of 1,226 patients, that at the time of enrolment, 44% were receiving only oral medications and 31% a prostacyclin combined with other agents. PAH-specific medications included 9% calcium channel blockers, 44% prostacyclin analogue, 47% ERAs and 46% PDE-5 inhibitors; 47% of patients were being treated with monotherapy. Eight percent of patients were receiving no PAH-specific medications.15
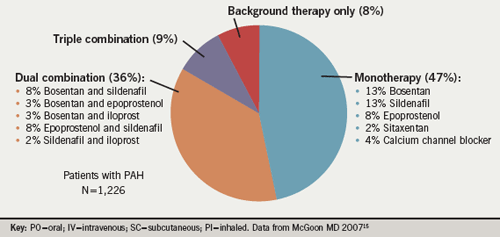
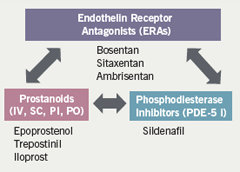
He commented that more than half of the patients were receiving combination therapy although there are as yet insufficient data to support this. The number of drugs and therefore the number of potential combinations of drugs that could be used is increasing rapidly (figure 2). The cost of combination therapy is likely to compound the challenges faced by physicians managing PAH patients, in Professor Hoeper’s view.
Findings from a small study of nine patients with idiopathic PAH from his centre16 showed that adding sildenafil to bosentan improves six-minute walk distance. In a similar study from Johns Hopkins University17 in patients with idiopathic PAH and in others with scleroderma, benefit was shown in the latter patients but not in the idiopathic cohort, suggesting that the PAH population is becoming increasingly complex and that not all forms of PAH may respond similarly to medical therapy. Also, new treatment options are rapidly emerging but comparative data are lacking. He believes that many questions will remain unanswered for some time.
“Treatment goals are becoming more ambitious year by year, and this is one of the reasons why combination therapy is now increasingly used. As PAH therapy is more complex than ever, this makes a strong case to treat these patients in experienced centres,” he said.
Conflict of interest
MMH has received lecturing fees and consultancy honoraria from Actelion, Bayer, Encysive, GSK, LungRx and Pfizer.
References
- Elliott GC. Chest 2007; 132: 631S.
- Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension. Eur Respir J 2007;30:1103–10.
- Gibbs JSR. Consensus statement on the management of pulmonary hypertension in clinical practice in the UK and Ireland. Heart 2008;94:1–41.
- Galie N, Ghofrani HA, Torbicki A et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–57.
- www.emea.europa.eu/humandocs/PDFs/EPAR/tracleer/H-401-PI-en.pdf
- www.emea.europa.eu/humandocs/PDFs/EPAR/thelin/H-679-PI-en.pdf
- www.emea.europa.eu/humandocs/PDFs/EPAR/volibris/H-839-PI-en.pdf
- Rubin LJ, Badesch DB, Barst RJ et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903.
- Barst RJ, Langleben D, Badesch D et al: STRIDE-2 Study Group. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol 2006;47:2049–56.
- Galie N, Olschewski H, Oudiz RJ et al. Ambrisentan for the treatment of pulmonary arterial hypertension. Results of the ARIES Study 1 and 2. Circulation 2008;117:3010–19.
- Johnson RS, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur Respir J 2006; 28:999–1004.
- Murphey LM, Hood EH. Bosentan and warfarin interaction. Annals Pharmacotherapy 2003;37:1028–31.
- Waxman AB. A review of sitaxsentan sodium in patients with pulmonary arterial hypertension. Vasc Dis Risk Manage 2007;3:151–7.
- Paul GA, Gibbs JSR, Boobis AR et al. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol 2005;60:1007–12.
- McGoon MD. REVEAL Registry: treatment history and treatment at baseline. Chest 2007;132:631S.
- Hoeper MM, Faulenbach C, Golpon H et al. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J 2004;24:1007–10.
- Mathai SC, Girgis R, Fisher MH et al. Addition of sildenafil to bosentan monotherapy in idiopathic pulmonary arterial hypertension. Eur Respir J 2007;29:469–75.
