Professor Nazzareno Galiè, University of Bologna, Italy, outlined the pathophysiological mechanisms of pulmonary arterial hypertension (PAH). These are initiated by the progressive obstructive changes of the pulmonary resistance vessels which lead to the increase in afterload of the right ventricle (RV). In turn, this responds with functional and structural adaptations, leading ultimately to heart failure and death.1 The hope is to have some treatment to help in reverse remodelling. Over the past 15 years or so there have been 22 randomised controlled studies, using a variety of PAH agents. He showed some ‘hypothesis-generating’ data from trials, using historical controls (prior to 1992) and then including patients treated with prostacyclin and more recently (after 2000) with oral agents. Although these patients are not identical or comparable at baseline, it appears that survival of patients who are referred to us today is much better than survival 15 or more years ago,2 said Professor Galiè.
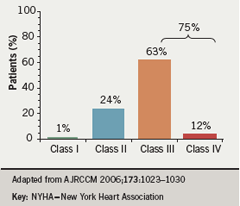
Despite greater awareness of PAH in recent years and the availability of targeted therapies, the majority of patients are at an advanced symptomatic stage by the time they are diagnosed.3 A French registry of 674 patients has highlighted that 75% of patients were in New York Heart Association (NYHA) Class III or IV at presentation (figure 1).3
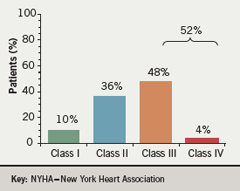
Clinical investigations to date have focused largely on these more compromised patient populations. However, some recent studies have included patients in the early symptomatic stages of the disease. This is supported by findings from the University of Bologna pulmonary vascular diseases centre (figure 2) showing in recent months (“probably because there is more attention to the disease”), that referral of patients in the early symptomatic stages of the disease appears to be increasing, said Professor Galiè. Studies with epoprostenol show significantly improved survival rates when patients are treated at NYHA class III compared to class IV.4
EARLY and…
He presented further data from trials including the EARLY study, showing benefit of treatment in less compromised individuals in World Health Organization (WHO) Functional Class (FC) I and II. EARLY was a double-blind, randomised controlled trial of six months’ treatment with bosentan (n=93) or placebo (n=92) in patients with WHO FC II PAH.5 The primary end points were pulmonary vascular resistance (PVR) at six months (expressed as percentage of baseline) and change from baseline to month six in six-minute walk distance. Results showed the mean PVR at six months was 83.2% of baseline value with bosentan and 107.5% with placebo (p=0.0001); mean six-minute walk distance increased by 11.2 m with bosentan and decreased by 7.9 m with placebo, giving a non-significant mean treatment effect of 19.1 m (p=0.0758).5 Significant benefits were seen, with reductions in pulmonary vascular resistance, in those patients who had haemodynamic assessments. More patients remained stable, without signs of clinical deterioration, in the bosentan group than in the placebo group (effect of bosentan on time to clinical worsening p=0.0114, log rank test).
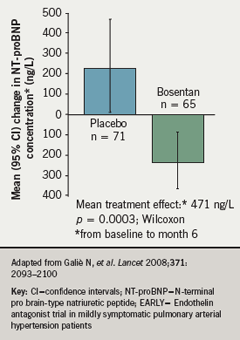
Exploratory end points in EARLY also included change from baseline to month six in N-terminal-prohormone brain natriuretic peptide (NT-proBNP). This too improved significantly, reflecting haemodynamic benefit, in the bosentan-treated patients (p=0.0003, Wilcoxon) (figure 3).5
Thirteen percent of patients in the bosentan group reported serious adverse effects (SAE), most commonly syncope; the SAE rate was 9% with placebo, the most common problem being right ventricular failure. Two deaths occurred during the study period, one in each patient group. Increases in aminotransferases >3x ULN occurred in 13% of bosentan-treated patients, compared to 2% in the placebo group.
…ARIES studies reviewed
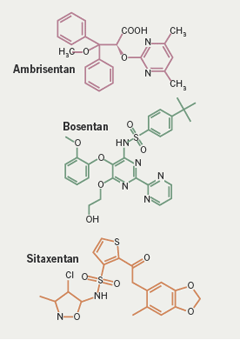
Professor Galiè then presented results from the ARIES studies with ambrisentan.6 This compound is structurally different to the other ERAs, bosentan and sitaxentan – it is a propanoic acid class molecule rather than a sulfonamide (figure 4).7
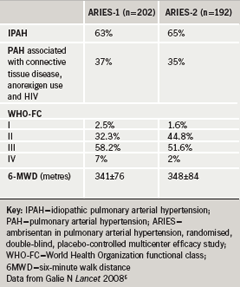
Ambrisentan is a selective endothelin A receptor antagonist which is administered orally once daily for treatment of PAH. The drug has been investigated in the recently reported ARIES-1 and ARIES-2 studies. ARIES-1 was conducted predominantly in North America, and ARIES-2 conducted mainly in Europe. The studies were concurrent, double-blind, and placebo-controlled. They randomised 202 and 192 PAH patients, respectively, to ambrisentan 5 or 10 mg (ARIES-1) or ambrisentan 2.5 or 5 mg (ARIES-2) orally once daily for 12 weeks. (Only the 5mg and 10mg doses are licensed for treatment of PAH.)
The primary end point for each study was change in six-minute walk distance from baseline to week 12. Clinical worsening, WHO FC, Short Form-36 Health Survey score, Borg dyspnoea score and B-type natriuretic peptide (BNP) concentrations were also assessed. In addition, a long-term observational extension study was also performed. Table 1 shows the baseline characteristics of patients in the studies: the majority of patients had idiopathic PAH and about 40% of patients were in the early symptomatic stages, FC I or II.6
Results showed that the six-minute walk distance increased significantly in all ambrisentan-treated groups.6 The mean placebo-adjusted treatment effects were 31 m and 51 m in ARIES-1 for ambrisentan 5 and 10 mg, respectively; and 32 m and 59 m in ARIES-2 for ambrisentan 2.5 and 5 mg, respectively. In patients completing 48 weeks of ambrisentan monotherapy (n=280), the improvement from baseline was 39 m. Time to clinical worsening did not improve significantly in ARIES-1 but a statistically significant improvement was observed in ARIES-2. Professor Galiè explained thathe pt, in his view, this difference was exclusively related to the difference in clinical worsening among the placebo groups of ARIES-1 and ARIES-2, which had different rates of hospitalisations at 3% and 14%, respectively. He believes that there is a geographical difference in attitude towards hospitalisations in Europe and the US, and in his view it is easier to hospitalise patients in Europe compared to the United States. There was a significant improvement in WHO FC in the ARIES-1 patients receiving ambrisentan (p=0.036), while a similar but non-significant trend was seen in ARIES-2 (p=0.117).Also in ARIES-1 and ARIES-2, there was a reduction in BNP compared to placebo for all doses of the drug (p=0.003) and there was a significant improvement in quality of life in ARIES-2.
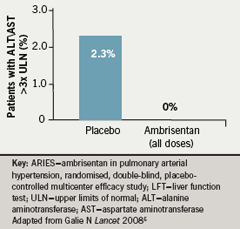
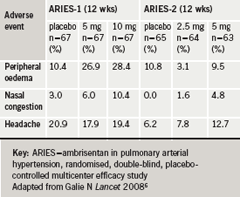
Interestingly, in the three months of this study no increases in liver enzymes more than three times the upper limit of normal were observed in the ambrisentan groups, but there was an increase in 2.3% of placebo patients (figure 5)6. Peripheral oedema, headache and nasal congestion are all peripheral effects which may be related to vasodilatation;8 they tended to be more frequent in the ambrisentan-treated patients (table 2)6. Twenty-two patients (16.7%) in the placebo groups and 25 patients (9.6%) in the combined ambrisentan group had at least one serious side effect. There were six deaths (4.5%) on placebo and four (1.5%) in the combined ambrisentan groups, none of them judged to be causally related to the study drug by the investigators.6
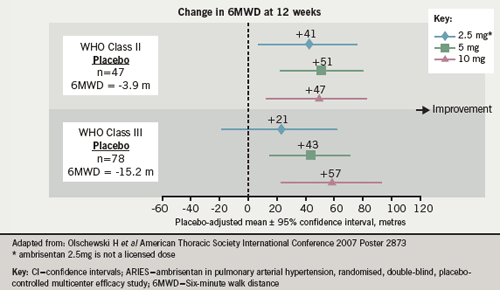
Professor Galiè then described a pre-specified combined analysis of the ARIES-1 and -2 studies.9 He said this confirmed that there is a dose response improvement in exercise capacity at 12 weeks with ambrisentan. The combined analysis also showed a statistically significant improvement in time to clinical worsening (71% relative risk reduction) for the three doses of ambrisentan (2.5, 5 and 10 mg once daily) relative to placebo: this distinction in time to clinical worsening between the treatment groups was not dose-related.9 Sub-group analysis also showed that the improvement in exercise capacity was very similar in both FC II and III patients, with improvements in 6MWD at 12 weeks of between 43% and 57% for ambrisentan 5mg and 10mg doses (figure 6).10 He presented open-label data on one-year incidence of aminotransferase abnormalities showing >3 x ULN rates of 2.8% and > 5 x ULN of 0.5%.
Summarising, Professor Galiè said that the evidence is now sufficient to justify the treatment of early symptomatic (class II) patients, thereby preserving functional capacity.
Treatment delays, even of a few months, may decrease the potential benefit patients can derive from drugs. “We have to start treating these patients with pulmonary hypertension as soon as we have a reliable diagnosis,” Professor Galiè concluded.
Conflict of interest
NG has participated in advisory board activities for Actelion, Pfizer, United Therapeutics, Eli-Lilly, Bayer-Schering, Encysive and GSK.
References
- Galie N, Manes A, Palazzini M et al. Pharmacological impact on right ventricular remodelling in pulmonary arterial hypertension. Eur Heart J 2007; 9: H68-H74.
- Sitbon O. ERS 2007: abstract 1593 (manuscript submitted).
- Humbert M, Sitbon O, Chaouat A et al. Pulmonary arterial hypertension in France : results from a national registry. Am J Resp Crit Care Med 2006; 173: 1023-30.
- Sitbon O, Humbert M, Nunes H et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780-8.
- Galie N, Rubin LJ, Hoeper MM et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093-100.
- Galie N, Olschewski H, Oudiz RJ et al. Ambrisentan for the treatment of pulmonary arterial hypertension. Results of ARIES 1 and 2. Circulation 2008; 117: 3010-19.
- Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag 2007; 3: 11-22.
- Cho S, Atwood JE. Am J Med 2002; 113: 580-6.
- Galie N. American Thoracic Society International Conference 2007, Poster 3192.
- Olschewski H. American Thoracic Society International Conference 2007, Poster 2873.
