Regular physical activity for secondary prevention in cardiovascular disease has many well-recognised benefits, with declines in physical activity being associated with worsening cardiovascular disease, suboptimal treatment or worsening comorbidities that might be rectified by early intervention. Most cardiovascular implantable electronic devices (CIED) now have the ability to detect, analyse and interpret physical activity data through an inbuilt accelerometer. Currently, these data are not being utilised to their full potential. We present three cases that demonstrate some of the possible uses of CIED-collected physical-activity data. These data have the potential to detect a deteriorating patient, to monitor the effects of an intervention, and/or provide motivational feedback to a patient. However, for the data to be used in this manner in the future, greater transparency from manufacturers and robust validation studies will be needed.
Background

One of the consequences of cardiovascular disease may be a limitation of physical activity, as a result of pain (e.g. in patients with angina) or inadequate cardiac output (e.g. in patients with heart failure). While the benefits of regular physical activity for secondary prevention in cardiovascular disease are well recognised,1 declines in physical activity may indicate a change in clinical status of a patient, whether this is in relation to cardiovascular diseases, such as heart failure,2 or the more general wellbeing of the patient. Thus, one measure of disease severity is to assess the objective exercise capability of patients with tests, such as an exercise stress test, or by patient reported measures in heart failure, such as the New York Heart Association (NYHA) scale. However, these tests are performed irregularly, may be costly to perform, and provide a single snapshot in time. Longitudinal, continuous measurement of physical activity may, therefore, provide an opportunity for early signs of worsening cardiovascular disease, suboptimal treatment or worsening comorbidities that might be rectified by early intervention.
Patients with the most severe cardiac disease may present the biggest opportunity for physical-activity monitoring and gain the most from early intervention. These patients will typically be fitted with cardiovascular implantable electronic devices (CIEDs), including implantable cardioverter defibrillators (ICDs), cardiac resynchronisation therapy (CRT) and pacemakers. Most of the CIEDs implanted in patients can collect and record physical activity data through built-in accelerometers, of which the primary function is to provide rate responsive pacing (adapting heart rate to physical activity). Piezoelectric crystal sensors detect changes in body motion as the patient moves, generating an electrical signal proportional to patient movement.3 These data are collected irrespective of rate response pacing and are available for interpretation at routine clinic visits or via remote monitoring of patients’ devices. The data typically provided by CIEDs are the amount of time spent being physically active expressed as number of hours per day.
There have been several studies4-8 that have used physical-activity data collected by CIEDs to provide information on functional status and prognostic information. However, anecdotal evidence suggests that these data are not used to their full potential and are currently considered an additional extra, rather than standalone data, perhaps due in part to the lack of validation studies performed in diverse patient samples and the limited information available from manufacturers about how devices detect, analyse and interpret the activity data. As well as having prognostic and clinical value, physical-activity data collected from CIEDs also represent a missed opportunity to feed back to patients about the level of physical activity they are undertaking. It has been shown that physical-activity monitors are an effective facilitator to increase physical activity9 and reduce sedentary time.10 Additionally, feedback from physical-activity monitors is suggested to motivate behaviour change,11 demonstrating the potential of feedback from physical-activity monitoring to increase physical activity. This case series will highlight how physical activity monitored through CIEDs is associated with patient health and wellbeing and, thus, demonstrate the potential uses of physical activity monitoring in this group of patients, whether to identify deteriorating patients or to monitor the effects of an intervention.
Case presentations
Patient A
Patient A is an 85-year-old man with a past medical history of previous coronary artery bypass grafting (CABG), left ventricular systolic dysfunction (LVSD), previous atrial fibrillation (AF) (successfully cardioverted), mild-to-moderate aortic stenosis and an implanted cardiac resynchronisation therapy pacemaker (CRT-P). Despite his age, patient A lived a very active lifestyle, working as a joiner until recently, walking more than two miles per day and also caring for his wife.
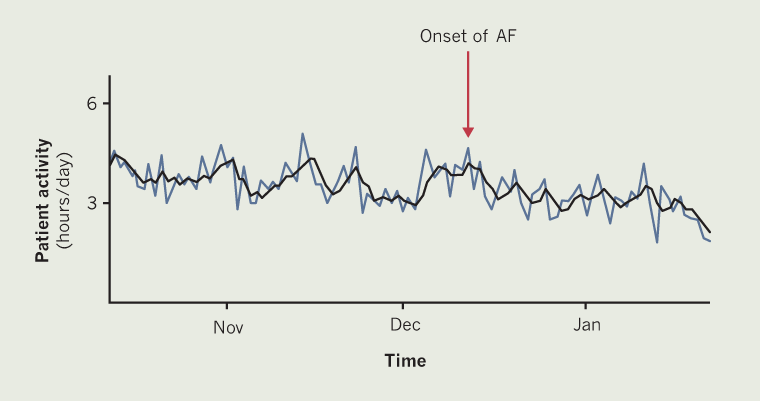
Remote monitoring data collected from the CRT-P device showed that, on average, he was classed as being active for between ~3.1 and ~4.3 hours per day between October and December (figure 1). This activity can be seen to gradually decline to an average activity of between ~2.0 and ~3.4 hours per day – an overall reduction of approximately 1.1 hours per day. This reduction coincided with the patient feeling more breathless and less able to do daily activities, including caring for his wife, resulting in an increased care package being organised. It was found that the decrease in physical activity and new onset of symptoms were associated with new onset of AF accompanied by increased respiration rate. These data could represent a missed opportunity for an earlier intervention in this patient. These findings were passed on to the specialist heart failure nurse and consultant for discussion about starting amiodarone.
Patient B
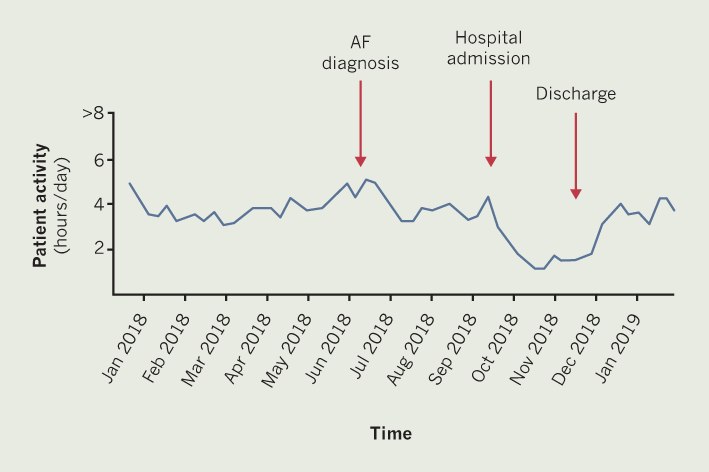
Patient B is a 92-year-old man with a past medical history of severe LVSD, hypertension, lacunar stroke with right-sided weakness and a CRT-P. In April 2018, patient B was well, in sinus rhythm and ‘enjoying’ life. In July 2018, he was found to be in AF, of which he was symptomatic, this was successfully cardioverted at the beginning of September 2018. There was an associated small decrease seen in physical activity at this time (figure 2). A further decrease in physical activity can be seen from the end of September, this was due to admission to hospital for hypotension-induced dizziness, vomiting and diarrhoea. At this time, a diagnosis of dementia was being investigated and patient B was transferred to a community hospital for one month while awaiting a care package to be put in place. Patient B’s hospital admission correlates with the lowest amount of physical activity seen in figure 2. Following discharge home, the level of physical activity begins to increase.
Patient C
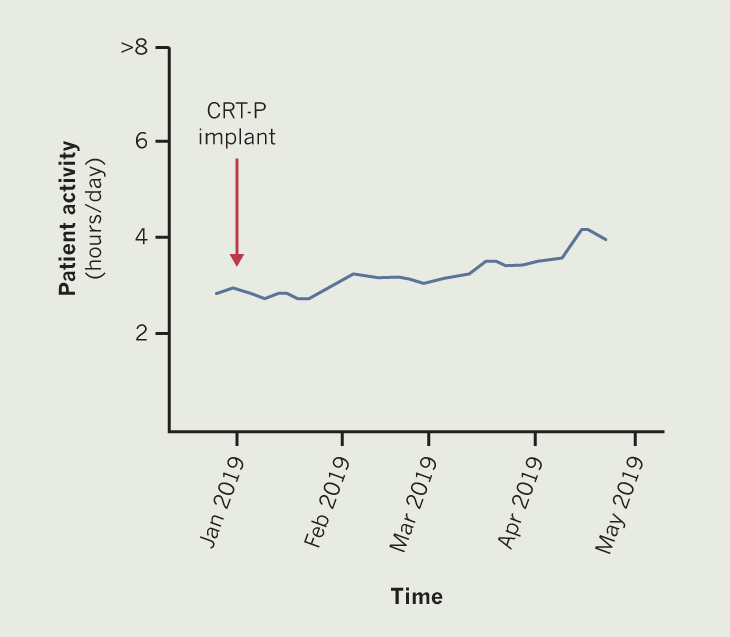
Patient C is a 95-year-old man with severe LVSD, AF and CRT-P. Prior to CRT-P implant in January 2019, patient C reported being able to walk around 200 yards (~180 m), before having to stop due to breathlessness. Following an upgrade of his pacemaker to a CRT-P, the patient reported an increase in exercise tolerance to around 300 yards (~270 m). Exercise tolerance increased further following optimisation of CRT pacing and increasing his dose of beta blocker. By March 2019, patient C reported being able to walk >500 yards (~460 m), with the limiting factor being back pain rather than breathlessness. Figure 3 demonstrates this general increase in hours of being physically active.
Discussion
All three cases presented here demonstrate how physical-activity monitoring through CIEDs could be used to influence clinical decision-making, identify deteriorating patients and monitor the effects of an intervention. Patient A represents a potential missed opportunity to identify a deteriorating patient sooner. If the patient’s normal levels of physical activity were known or documented somewhere then the decline in physical activity, which coincided with the patient’s breathlessness and onset of AF, may have been highlighted earlier. Earlier detection would have allowed for earlier medical intervention and perhaps removed the need for an increased care package.
Patient B clearly demonstrates the difficulties faced in remaining physically active while in hospital. There is increasing recognition that deconditioning associated with physical inactivity during hospitalisation can result in a range of adverse effects, including increased frailty,12 functional decline,13 and development of disability in activities of daily living.14 All of these factors could lead to longer hospital stays and delays in discharge, to increased care packages then needed at home. If the physical-activity monitoring from CIEDs was fed back to the patient and/or the medical and nursing team, this could increase the motivation for remaining physically active while in hospital. Walking in hospitals is associated with a decreased length in stay,15 which can only be seen as a benefit for both the patient and the health service. It would have also been of benefit to the patient and clinicians if, after discharge, data about the patient’s increasing activity was fed back. Activity data would not only have provided motivation to continue exercising, but also demonstrated improvements in the patient’s wellbeing.
Patient C is an example of how physical-activity monitoring can be used to monitor the effects of an intervention, this information would be useful for both clinicians and patients alike. However, these data are only available to clinicians and data are not currently provided directly to the patient.16 Even though the data are fed back on a general basis at clinic visits, daily or weekly updates direct to the patient could increase motivation and further increase activity.
The three cases presented here demonstrate some of the possible uses of physical-activity monitoring from CIEDs. However, it is important to note some of the limitations of the data, and the need for further studies, before the full benefits of the data can be utilised. There is currently limited information available from device manufacturers about how accelerometers detect, analyse and interpret activity data. Available information suggests that activity thresholds and processing algorithms differ considerably between manufacturers. This could lead to possible problems if, for example, trying to determine thresholds for when a patient is considered to be deteriorating or when an intervention has worked. If physical-activity data from CIEDs were to be used as a risk-stratification tool, it would likely be as a multi-parameter tool rather than using physical activity as a standalone measure. Before physical activity data from CIEDs is used as feedback for patients, validation studies are needed in diverse patient samples and over a range of activities to prove that the data being collected are comparable with wearable physical-activity devices.
Key messages
- Physical-activity monitoring from cardiovascular implantable electronic devices (CIEDs) may be useful in detecting a deteriorating patient or monitoring an intervention
- Direct patient feedback of physical-activity monitoring data collected from CIEDs could increase patient motivation to maintain or improve physical activity
- Validation studies in diverse patient samples and across a range of activities are needed
- Manufacturers need to provide more information surrounding detection, analysis and interpretation of physical-activity monitoring data from CIEDs
Conflicts of interest
None declared.
Funding
There was no specific funding for this project. DJM, OMG and SJL are supported by the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB). KC is supported by the Inverness and Highland City-Region Deal (UK Government). DC is supported by Highland and Islands Enterprise (grant number HMS 9353763).
Patient consent
Written consent was obtained from the three patients featured in this paper.
References
1. Darden D, Richardson C, Jackson EA. Physical activity and exercise for secondary prevention among patients with cardiovascular disease. Curr Cardiovasc Risk Rep 2013;7:411–16. https://doi.org/10.1007/s12170-013-0354-5
2. Faggiano P, D’Aloia A, Gualeni A, Brentana L, Dei Cas L. The 6 minute walking test in chronic heart failure: indications, interpretation and limitations from a review of the literature. Eur J Heart Fail 2004;6:687–91. https://doi.org/10.1016/j.ejheart.2003.11.024
3. Kaszala K, Ellenbogen KA. Device sensing. Circulation 2010;122:1328–40. https://doi.org/10.1161/CIRCULATIONAHA.109.919704
4. Braunschweig F, Mortensen PT, Gras D et al. Monitoring of physical activity and heart rate variability in patients with chronic heart failure using cardiac resynchronization devices. Am J Cardiol 2005;95:1104–07. https://doi.org/10.1016/j.amjcard.2004.12.069
5. Kadhiresan VA, Pastore J, Auricchio A et al. A novel method – the activity log index – for monitoring physical activity of patients with heart failure. Am J Cardiol 2002;89:1435–7. https://doi.org/10.1016/S0002-9149(02)02364-0
6. Kawabata M, Fantoni C, Regoli F et al. Activity monitoring in heart failure patients with cardiac resynchronization therapy. Circ J 2007;71:1885–92. https://doi.org/10.1253/circj.71.1885
7. Whellan DJ, Ousdigian KT, Al-Khatib SM et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations. J Am Coll Cardiol 2010;55:1803–10. https://doi.org/10.1016/j.jacc.2009.11.089
8. Snipelisky D, Kelly J, Levine JA et al. Accelerometer-measured daily activity in heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e003878. https://doi.org/10.1161/CIRCHEARTFAILURE.117.003878
9. Kang M, Marshall SJ, Barreira TV, Lee J-O. Effect of pedometer-based physical activity interventions. Res Q Exerc Sport 2009;80:648–55. https://doi.org/10.1080/02701367.2009.10599604
10. Qiu S, Cai X, Ju C et al. Step counter use and sedentary time in adults: a meta-analysis. Medicine (Baltimore) 2015;94:e1412. https://doi.org/10.1097/MD.0000000000001412
11. Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA 2015;313:459. https://doi.org/10.1001/jama.2014.14781
12. Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci 2011;66A:1238–43. https://doi.org/10.1093/gerona/glr142
13. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–23. https://doi.org/10.7326/0003-4819-118-3-199302010-00011
14. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004;292:2115. https://doi.org/10.1001/jama.292.17.2115
15. McCullagh R, Dillon C, Dahly D, Horgan NF, Timmons S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas 2016;37:1872–84. https://doi.org/10.1088/0967-3334/37/10/1872
16. Nash IS. It’s my heart. Circulation 2018;137:4–6. https://doi.org/10.1161/CIRCULATIONAHA.117.031392
