Introduction
Antithrombotic therapies including anticoagulation and antiplatelet agents are immensely powerful medical treatments, which prevent morbidity and mortality for a variety of indications in patients of all ages. However, the major drawback is the risk of bleeding, particularly major bleeding. Management of bleeding in patients who are therapeutically anticoagulated is therefore an important subject that transcends numerous medical specialities.
In this module, we will discuss the treatment options, the evidence behind these options, and we will detail the practical management and highlight specific actions required in a number of clinical situations.
Urgent versus non-urgent management
The most important distinction that will guide management is whether anticoagulation needs to be reversed within minutes, or whether we can afford to wait. Urgent reversal is required when there is either major bleeding or prior to urgent surgery or procedures, which cannot be delayed and cannot be performed whilst a patient is therapeutically anticoagulated.
This will require the administration of a ‘reversal agent’ whereas slower approaches either require time for the drug to wear off naturally or in the case of vitamin K for warfarin, the manufacture of endogenous clotting factors.
When to reverse: the importance of risk balance
The decision to stop antithrombotics or administer a reversal agent should not be taken lightly. This should be a carefully considered decision based on an individualised risk balance assessment, which should take into account:
- the antithrombotic drug and its pharmacokinetics
- urgency of reversal required
- severity of bleeding or risk of bleeding if prior to a procedure
- indication for anticoagulation and risk of thrombosis.
In patients at particularly high risk of thrombosis, many patients and clinicians will accept an increased risk of bleeding. For example, reversal of anticoagulation in a patient with a metallic mitral valve who has had a previous stroke is a different prospect to a patient who had a deep vein thrombosis 10 years ago. Ultimately, the decision is a clinical one, often guided by a haematologist.
What is meant by ‘reversal’?
Before we continue, it is worth considering this question. ‘Reversal’ implies complete negation of the anticoagulant effect of a drug and the restoration of normal haemostasis. Switching off the effect of a drug does not necessarily require the administration of an antidote and in many cases, simply stopping the anticoagulant is sufficient.
Administration of a reversal agent during the care of an anticoagulated patient who is bleeding is only a small part of the patient’s care and is not a panacea. Optimal care of such patients requires expert management including resuscitation, blood transfusion and interventional procedures.
Finally, although it is intuitive to think that administration of a reversal agent would improve the outcome of an anticoagulated patient who is bleeding, there are no randomised clinical trials which support superiority over placebo. This is an important point as reversal of anticoagulation may be harmful in some situations (see ‘Risks of reversal of anticoagulation’ section below) so this lack of evidence must guide cautious practice. Clinicians often overestimate benefits and underestimate the harms of treatments1 and medicine is full of examples where intuitive practice has been proven ineffective or even harmful.2 Indeed, administration of platelets for patients on antiplatelet agents who have spontaneous intracerebral haemorrhage has recently been shown to be harmful3 (see ‘Reversal of antiplatelet agents’ section below).
Reversal agents
A number of reversal agents are available in the UK, and these are summarised below. It is important to remember that in situations where there is non-major bleeding, it is usually more appropriate to allow the drug to wear off on its own rather than administer a reversal agent. However, in the 1–3% of patients who do experience major bleeds, reversal should be considered.
- Andexanet alfa: decoy factor Xa molecule with no enzymatic activity that competes for and reversibly binds anti-FXa inhibitors.
- Idarucizumab: monoclonal antibody that binds dabigatran.
- Four factor prothrombin complex concentrate (4F-PCC): a blood product containing concentrated clotting factors II, VII, IX, and X.
- Protamine sulfate: positively charged protein molecule that binds negatively charged heparin.
- Vitamin K: replenishes depleted stores of reduced vitamin K, enabling completion of production of factors II, VII, IX, and X.
- Platelet transfusion: for reversal of the effects of antiplatelets.
- Activated charcoal: adsorbs a wide range of oral drugs, can be effective if used within two to four hours of ingestion.
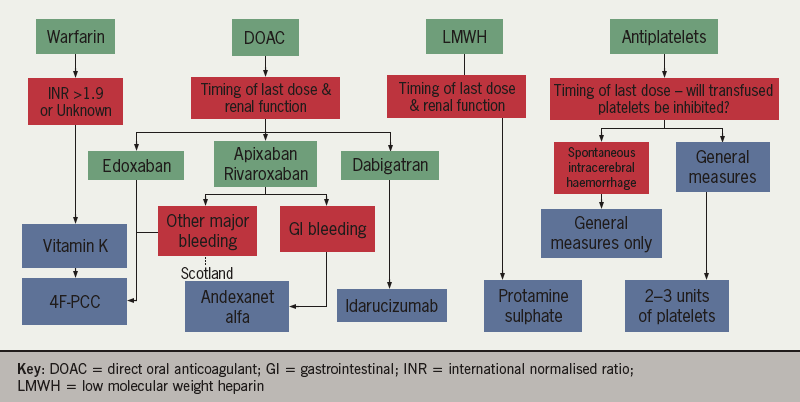
The preferred choice of reversal agent to use in different clinical situations, according to UK approvals, is shown in table 1. Figure 1 summarises the reversal agents for antithrombotic therapy.
Table 1. Preferred choice of reversal agent according to UK approvals
| Gastrointestinal bleeding | Intracranial bleeding | Other major bleeding | Urgent surgery | |
|---|---|---|---|---|
| Dabigatran | Idarucizumab | Idarucizumab | Idarucizumab | Idarucizumab |
| Apixaban and rivaroxaban | Andexanet alfa | 4F-PCC* Andexanet alfa (Scotland) | 4F-PCC* Andexanet alfa (Scotland) | Expectant management – only treat if bleeding – 4F-PCC |
| Edoxaban | 4F-PCC* | 4F-PCC* | 4F-PCC* | Expectant management – only treat if bleeding – 4F-PCC* |
| Heparin/LMWH | Protamine sulfate | Protamine sulfate | Protamine sulfate | Consider protamine sulfate or expectant management |
| Vitamin K antagonist | Vitamin K + 4F-PCC | Vitamin K + 4F-PCC | Vitamin K + 4F-PCC | Vitamin K + 4F-PCC |
| *4F-PCC use for DOACs is off-licence Key: LMWH = low-molecular weight heparin |
||||
Definition of major bleeding
A useful definition of major bleeding is the International Society of Thrombosis and Haemostasis definition:4
-
- Fatal bleeding.
and/or
-
- Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome.
and/or
- Bleeding causing a fall in haemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells.4
However, in practice, major bleeding is often considered when there is bleeding with haemodynamic compromise. It is not necessary to wait for the haemoglobin result or until red cells have been transfused.
Risks of reversal of anticoagulation
- Risk of thrombosis associated with withdrawal of anticoagulant effect
- may be heightened due to abrupt reversal rather than allowing natural tapering of effect.
- Risk of thrombosis due to reversal agent itself
- increased thrombin generation compared to baseline may be seen following administration.
- Risk of thrombosis due to not restarting anticoagulation appropriately
- Generic adverse effects of drug and blood product exposure
- infection
- allergic reaction.
- High financial cost.
Reversal of warfarin
The risk of major bleeding for most patients on warfarin ranges between about 1–5% per year but may be higher in some groups including the elderly. It is also higher when the international normalised ration (INR) is supratherapeutic.5,6
This section is also applicable to occasional patients taking alternative vitamin K antagonists e.g. sinthrone.
Evidence for warfarin reversal
Replenishing vitamin-K-dependent clotting factors in a warfarinised patient who is bleeding would seem intuitive. However, there has never been a randomised controlled trial comparing reversal with placebo. Despite this, a retrospective study suggested a benefit for 4F-PCC and FFP over no treatment for intracranial haemorrhage.7 A prospective study found that PCC produces a more rapid and more complete correction of coagulopathy.8 Finally, a systematic review which included 5 randomised studies and 8 observational found that PCC likely improved clinical outcomes compared to FFP.9
4F-PCC, along with vitamin K is thus the standard of care for warfarinised patients who are bleeding.
Practical management
Reversal of the effect of warfarin in a patient with major bleeding requires:
- Administration of 25-50 units/kg four-factor prothrombin complex concentrate (4F-PCC).
- Administration of 5 mg intravenous vitamin K (phytomenadione) for enduring reversal.
F4-PCC is a product manufactured from blood that contains the vitamin-K-dependent clotting factors: II, VII, IX, and X at concentrations 25-times higher than plasma.10
As the half-life of warfarin is ~36 hours, and the half-life of factor VII is six hours (the shortest half-life of the factors in PCC), vitamin K is essential as it overcomes the effect of warfarin which is to deplete reduced vitamin K and thus prevent the manufacture of the vitamin-K-dependent clotting factors (specifically preventing gamma-carboxylation of precursors to these clotting factors).
For non-major bleeding, where there is an option to wait six to 12 hours for the effect, administration of 5 mg vitamin K alone, oral or intravenous is sufficient. Both modes of administration are as effective, but intravenous administration is thought to have a more rapid onset.11 When administered orally, the patient simply drinks the intravenous solution.
Administration of 4F-PCC
4F-PCC is available in all major UK hospitals as a lyophilised product, which needs to be mixed up with water. In the UK, two proprietary products are available: Beriplex and Octaplex, which contain comparable amounts of the clotting factors.12,13 Don’t confuse Octaplex with Octaplas, which is a pooled solvent-detergent FFP product.
- route: intravenous (tables 2 and 3)
- dose: calculated by body weight (tables 2 and 3)
Beriplex (reconstituted at 25 international units [IU]/ml)
Table 2. Dosing of Beriplex
| Pre-treatment INR | 2.0–3.9 | 4.0–6.0 | > 6.0 |
|---|---|---|---|
| Approximate dose ml/kg body weight | 1 | 1.4 | 2 |
For calculations, patients >100 kg should have weight capped at 100 kg. Maximum single dose is 5,000 IU (200 ml)
Administered no faster than 8 ml/min.
Octaplex (reconstituted at 25 IU/ml)
Table 3. Dosing of Octaplex
| Initial INR | 2–2.5 | 2.5–3 | 3–3.5 | > 3.5 |
|---|---|---|---|---|
| Approximate dose ml/kg body weight | 0.9–1.3 | 1.3–1.6 | 1.6–1.9 | > 1.9 |
Maximum single dose should not exceed 3,000 IU (120 ml).
Administered initially at 1 ml per minute then 2-3 ml per minute.
A comparison of the clotting factor content of Beriplex and Octaplex is shown in table 4.
Table 4. Clotting factor content of the 4F-PCC products Beriplex and Octaplex, and the clotting factor half-lives
| Beriplex | Octaplex | Half-life | |
|---|---|---|---|
| Factor II | 20–48 | 13–38 | 60–72 |
| Factor VII | 10–25 | 9–24 | 4–6 |
| Factor IX | 20–31 | 25 | 24 |
| Factor X | 22–60 | 18–30 | 48–72 |
| Protein C | 15–45 | 13–31 | 8 |
| Protein S | 12–38 | 12–32 | 30 |
Reversal of the anticoagulant effect of DOACs
The direct oral anticoagulants (DOACs) include the direct thrombin inhibitor dabigatran and the anti-FXa inhibitors: apixaban, edoxaban, and rivaroxaban.
The key, important differences between reversal of warfarin and the DOACs are:
- Peak anticoagulant effect of DOACs is one to four hours post-dose.
- Anticoagulant effect of DOACs naturally wears off quickly due to relatively short half-lives (see table 5). Half-lives are dependent on renal function.
- Standard laboratory tests of coagulation cannot generally be reliably used to determine whether a patient is clinically meaningfully anticoagulated.14
Table 5. Half-life of direct oral anticoagulants (DOACs) in patients with normal renal function (creatinine clearance >80 ml/min) and effect on standard laboratory tests of coagulation
| Approximate half-life (hours) | Normal result used to rule out clinically meaningful drug levels? | |||||||
|---|---|---|---|---|---|---|---|---|
| Creatinine clearance (ml/min) | Accurate quantification | aPTT | PT/INR | TT | ||||
| >80 | 50–80 | 30–50 | 15–30 | |||||
| Dabigatran | 12–17 | 17 | 19 | 28 | Dilute thrombin time or ecarin clotting time | No* | No | Yes |
| Apixaban | 12 | 14.5 | 17.5 | 17.5 | Calibrated anti-Xa assay | No | No | No |
| Edoxaban | 10–14 | 8.5 | 9.5 | 17 | Calibrated anti-Xa assay | No | No | No |
| Rivaroxaban | 5–9, (11–13 elderly) | 8.5 | 9.0 | 9.5 | Calibrated anti-Xa assay | No | No | No |
| *Normal aPTT will exclude therapeutic dabigatran levels if a sensitive reagent is used | ||||||||
As the half-lives are short, and accurate quantification of drug levels is usually not readily available in an emergency situation, the time from last dose should be considered.
Use of standard coagulation tests to determine whether therapeutic drug levels are present
Unlike warfarin, the standard laboratory tests of tests of coagulation – activated partial thromboplastin time (aPTT), prothrombin time (PT), and thrombin time (TT), are not reliable measures of whether a patient has meaningful levels of DOACs in their plasma. The exception to this is dabigatran, where a normal thrombin time rules out therapeutic drug levels. Whilst apixaban, edoxaban and rivaroxaban variably prolong these clotting times (predominantly the PT), normal aPTT, PT and TT do not exclude out therapeutic drug levels.15,16
Evidence for DOAC reversal

There are three important agents to be aware of:
- andexanet alfa
- idarucizumab
- prothrombin complex concentrate.
Andexanet alfa
Andexanet alfa is a specific reversal agent that is a decoy factor Xa molecule without enzymatic activity. It is administered via a bolus, followed by infusion, of a dose which produces excess drug to compete successfully against endogenous factor Xa to reversibly bind anti-FXa drugs including apixaban and rivaroxaban.
In randomised phase III studies in healthy volunteers taking apixaban and rivaroxaban, andexanet alfa was shown to wholly reverse the drugs’ effect on anti-factor Xa activity and restore thrombin generation compared with placebo.17 Clinical evidence comes from a phase IIIb/IV multicentre, prospective, open-label, single-arm trial, ANNEXA-4.18 In this trial, 352 patients with acute major bleeding were studied – 64% with intracranial bleeding and 26% with gastrointestinal bleeding. The vast majority were taking apixaban and rivaroxaban, hence the approval is only for these two drugs. Notable exclusions in the trial were:
- last dose of drug >18 hours prior
- Glasgow coma score (GCS) <7
- estimated cerebral haematoma volume >60 ml
- expected survival <one month.
28% patients had either subtherapeutic drug levels (results were not available at the time of treatment) or were later adjudicated to not have had severe enough bleeding, and were only included in the safety analysis, not the efficacy analysis.
The study had two co-primary outcomes, haemostatic efficacy at 12 hours and reduction in anti-Fxa activity:
- Proportion of patients with excellent or good haemostatic efficacy at 12 hours after the andexanet alfa infusion: 82%.
- Median reduction in anti-FXa activity: 92%.
- Reduction in anti-FXa activity was modestly predictive of haemostatic efficacy for intracerebral haemorrhage but not overall.
- 30-day mortality:14%.
- 30 day Incidence of thrombosis within 30 days: 10% (of whom 11 patients [3.1% of the total] had an event <six days after treatment.
It is therefore clear that andexanet alfa is an active drug with definite physiological effects. However, as ANNEXA-4 had no comparator arm, an effect on morbidity and mortality over and above stopping the drug and supportive management has not been demonstrated as yet. This is also true of PCC for DOAC reversal, and idarucizumab as discussed below.
Prothrombin complex concentrate
As andexanet alfa was not available for the DOACs when they were launched, prothrombin complex concentrate (PCC) has been used off-licence. Both three factor, and four factor PCCs are manufactured but four factor PCC contains higher concentration of factor VII and is the product used widely in the UK. From here on, when we refer to PCC, we mean 4F-PCC.
The use of PCC was initially supported by observations of increased thrombin generation and normalisation of prothrombin time in DOAC-treated healthy volunteers.19,20 Studies of PCC for prevention of skin biopsy-related bleeding in healthy volunteers treated with DOACs have shown mixed results.21,22
Subsequent, small observational studies have reported variable results but the nature of the data is such that PCC has no formal clinical evidence of benefit to support its use. Importantly, a large German registry study recorded 1,775 bleeding episodes of which 66 were major bleeds.23 Only six patients received PCC in this cohort and overall 30-day mortality was 10%, comparable with mortality in other observational studies and trials where patients were treated with reversal agents.
Additionally, it is entirely plausible that PCC may add to thrombosis risk as it contains activated clotting factors and can elevate thrombin generation from baseline for over 24 hours post-infusion.
Andexanet alfa versus PCC
Although studies in animal bleeding models have suggested a benefit for andexanet alfa over PCC in ideal conditions,24–26 no direct clinical comparisons of PCC versus andexanet alfa have been performed.27 A propensity-score matched comparison of patients in the UK registry study, ORANGE,28 who received PCC versus patients in ANNEXA-4 who received andexanet alfa, suggested a significant mortality benefit for andexanet alfa (14.6% vs. 34.1%) but there are major flaws in this conclusion, most importantly as there was no adjustment for bleeding severity.29
In conclusion, PCCs are used and are recommended in some bleeding guidelines, but this use is outside of the licence and the only clinical trials are observational, uncontrolled and small in size – there are no randomised trials. In contrast to PCCs, andexanet alfa is licensed for use in major bleeding, but the evidence for a clinical benefit on morbidity, mortality, or quality of life, either compared to supportive care or PCC, is at this time, lacking. A head-to-head study of andexanet alfa versus standard of care (PCC) is ongoing (ANNEXA-I)30 but is not expected to report until approximately 2023. Due to these shortcomings in the evidence, reversal agents should continue to be used with caution.
Idarucizumab
The monoclonal antibody Idarucizumab is available in the UK for urgent reversal of the effects of dabigatran either in a bleeding patient or prior to emergency surgery or urgent procedures. Its approval is based on a phase II, single arm study31 which showed, in patients who were bleeding:
- Median maximum percentage reversal of dabigatran: 100%.
- Bleeding cessation within 24 hours: 67.7%.
- Median time to haemostasis: 2.5 hours.
- 30-day mortality: 13.5%.
- 30-day incidence of thrombosis: 4.8%.
As for other reversal agents, the evidence is similarly limited to single-arm clinical trial data only; we are, again, lacking clear evidence of clinical benefit for idarucizumab. Dabigatran can also be removed by haemodialysis although this is unlikely to be logistically straightforward in an emergency setting.
Use of reversal agents for DOACs prior to urgent surgery or procedures
The REVERSE-AD trial, which led to the licensing of idarucizumab for dabigatran reversal included 503 patients, 301 of whom were bleeding and 202 of whom received the drug prior to an urgent procedure. Periprocedural haemostasis was described as normal in 93.4% and this led to its approval for this purpose.31
PCC and andexanet alfa have no such evidence and are not licensed for this purpose. This is reflected in international guidelines which recommend expectant management i.e. administration of a reversal agent only if there is serious bleeding.
Practical management: administration of DOAC reversal agents
Andexanet alfa
International guidelines generally recommend andexanet alfa as the preferred treatment for anti-FXa drug reversal.32 Despite this, and although its licence is for any life-threatening or uncontrolled bleeding, in England, andexanet alfa is only funded for gastrointestinal bleeding,33 whereas in Scotland it is funded as per the licence.34 Andexanet alfa is a notably high-cost drug33 and many NHS trusts are expected to institute strict guidelines to restrict its use to limited situations guided by ANNEXA-4 inclusion criteria.
Andexanet alfa is administered as an initial intravenous bolus followed by an infusion for up to two hours. It is given at either low or high dose depending on when the last dose of the drug was given (before or after eight hours).35
Table 6. Decision tool for determining whether andexanet alfa should be administered at low dose or high dose based on drug dose and time elapsed since most recent administration
| Last drug and dose | Time elapsed since last dose | Reversal dose required | |
|---|---|---|---|
| Apixaban | Rivaroxaban | ||
| ≤ 5 mg | ≤10 mg | Any | Low dose |
| > 5 mg / unknown | >10 mg / unknown | ≥ 8 hours | Low dose |
| < 8 hours / unknown | High dose | ||
Table 7. Dosing of andexanet alfa according to whether low dose or high dose is indicated
| Initial intravenous bolus | Continuous intravenous infusion | |
|---|---|---|
| Low dose | 400 mg at a target rate of 30 mg/min | 4 mg/min for 120 minutes (480 mg) |
| High dose | 800 mg at a target rate of 30 mg/min | 8 mg/min for 120 minutes (960 mg) |
Unlike warfarin reversal where, as long as adequate vitamin K is administered, the reversal is permanent, administration of andexanet alfa only induces a transient fall in drug levels, as the inhibition is reversible and the andexanet alfa half-life is short. As such, drug levels can increase again at the end of the infusion, particularly if the patient had recently taken the dose.18 The drug is not licensed for a second dose so if rebleeding occurs, alternative steps should be taken. However, it is thought that the three hours that the anti-FXa activity is reduced is sufficient for a stable clot to form at the site of bleeding. Also, it should be noted that the anti-FXa inhibitors are not fibrinolytic so should not affect the clot that forms.
Idarucizumab
Idarucizumab is available in pre-mixed vials containing 2.5 g in 50 ml. The recommended dose is 5 g as two 2.5 mg intravenous infusions over five to 10 minutes each or as a bolus.36
In some patients, the anticoagulant effect of dabigatran may rebound up to 24 hours after administration of idarucizumab, as can be demonstrated by prolongation of clotting tests (as measured by thrombin time, dilute thrombin time or Ecarin clotting time). A second dose of 5 g may therefore be considered in the following situations:
-
- Prolonged clotting times AND
-
- recurrence of clinically relevant bleeding
OR
- if potential re-bleeding would be life-threatening and prolonged clotting times are observed
-
- Prolonged clotting times AND
OR
- Patient requires a second emergency surgery/urgent procedure and have prolonged clotting times.
Prothrombin complex concentrate
As use of 4F-PCC for DOAC reversal is off-licence, there is no set dose. However, dosing options include:
- fixed dose of 2000 units
- 25-50 units/kg capped at 3000 units (Octaplex) or 5000 units (Beriplex).37
Administer as detailed in section 2.3.
Activated charcoal
Activated charcoal adsorbs a wide range of drugs and is an option for reversal of DOACs within two to four hours of ingestion.38–43 However, there are no trials demonstrating benefit in DOAC-associated bleeding.
Activated charcoal is given orally at a dose of 50 g.
Reversal of heparins
The important heparins to be aware of are:
- unfractionated heparin (UFH)
- low molecular weight heparin (LMWH)
- ultra-low molecular weight heparin (only available formulation is fondaparinux).
The reversal agent for UFH and LMWH is protamine sulfate. Protamines are small, nuclear proteins that replace histones late in spermatogenesis allowing for tighter DNA packaging in the mature sperm; protamine sulfate is derived from salmon sperm. Administration can be associated with severe hypotension and anaphylaxis.44 Vasectomised men commonly have anti-protamine, anti-sperm antibodies and are thus thought to be at higher risk of anaphylaxis when exposed to protamine sulfate. Diabetic patients who have received protamine bound insulin (which is used to extend the length of action of the insulin), can also have antibodies. Having said this, the data backing up this perceived increased incidence is limited to case reports.45
Unfractionated heparin
Usually, UFH is used as an intravenous infusion in specialist areas and has a short half-life of only 30–60 minutes. If urgent reversal is required, the effects can be fully reversed with protamine sulfate.
Dosing must be done carefully as protamine itself in gross excess acts as an anticoagulant. 1 mg will neutralise at least 100 international units of heparin. The infusion should be stopped and 25–50mg of protamine sulfate given. The maximum dose is 50 mg. As protamine is cleared more quickly than heparin, further doses may be required using the aPTT for guidance.44
Low molecular weight heparin
LMWHs exhibit decreased plasma protein binding compared to UFH. This gives both a wider therapeutic index, and decreased clearance leading to a substantially longer half-life than UFH of 3–5 hours. As with the anti-FXa inhibitors, normal standard laboratory tests of coagulation do not rule out therapeutic levels.46
Elimination occurs by two mechanisms: binding to endothelial cells and clearance by the reticuloendothelial system, and a mechanism involving mainly renal clearance.
Anti-FXa activity persists for over 12 hours
Reversal of LMWH with protamine sulfate is not as effective as for UFH – it provides only partial reversal of LMWH (about 60% as effective).47 As with the DOACs, the evidence base for its use is limited to observational studies only so there is no clear evidence of clinical benefit.
The longer half-life of LMWH compared to UFH should be taken into account when dosing and most guidelines recommend halving the dose after 8 hours post-dose have elapsed. After 12 hours post-dose, protamine is unlikely to be helpful but serious cases should be discussed with a haematologist.
For example:
A 65-year old man has been treated for a pulmonary embolism with a daily subcutaneous injection 18,000 units of dalteparin for the last five days. He has developed life-threatening gastrointestinal haemorrhage. The last dose of dalteparin was 10 hours ago and he has a creatinine clearance of 85 ml/min.
The protamine sulfate dose would therefore be calculated as 1 mg per 100 units:
(12,000 / 100) / 2 = 60 mg
However, remember, the dose is capped at 50 mg.
Due to on-going, slow absorption into the circulation from the subcutaneous injection, so the patient should be monitored and further doses or an infusion may be required.
Fondaparinux
Fondaparinux has a long half-life of about 17 hours and there are very limited data on measures that can be taken to reverse its effects; protamine sulfate is not effective. A handful of studies have examined the role of recombinant, activated factor VII (rFVIIa)48 and factor eight inhibitor bypassing activity (FEIBA) and have shown variable effects on thrombin generation.49 However, there is no accepted treatment and fondaparinux should not be used where there is a high risk of bleeding or a likelihood of the patient needing urgent procedures.
Theoretically, andexanet alfa will also have activity for reversal of LMWH and fondaparinux.41 However, due to very low numbers of LMWH-treated patients in ANNEXA-4 and no patients on fondaparinux, it is not licensed for this purpose.
Reversal of antiplatelet agents
The two most commonly used antiplatelet agents, aspirin and clopidogrel, are irreversible platelet antagonists. The manufacture of new platelets is therefore required to overcome their effects hence the five to seven day wash-out periods prior to procedures. Platelets transfused within six to 12 hours of the last dose will also become inhibited by antiplatelets and effects will be very limited.
This is yet another area where there is very limited evidence of benefit for any intervention. The 2012 British Society of Haematology guidelines recommend platelet transfusion for patients who continue to bleed when other haemostatic interventions have failed. However, this is a grade 2C recommendation (weak recommendation, weak evidence).43
In fact, there is evidence of harm of platelet transfusion in intracranial haemorrhage. The 2016 PATCH trial randomised 190 adults with spontaneous cerebral haemorrhage associated with antiplatelet therapy to receiver or not receive platelet transfusion. They found an increased risk of dependence or death at three months in those who received platelet transfusion.3
Important points to be aware of in this trial are:
- ~80% patients were taking aspirin only
- the remaining majority were taking aspirin + dipyridamole
- the intervention was 5 units of platelets for patients taking aspirin and 10 for patients taking aspirin and dipyridamole
- patients with subdural haemorrhage and traumatic haemorrhage were excluded.
Another option for patients who are bleeding on antiplatelets is desmopressin, which stimulates release of factor VIII and von Willebrand factor from the vascular endothelium. However, this is relatively contraindicated in patients with cardiovascular disease, which includes the majority of patients taking antiplatelets.
In conclusion, platelet transfusion can be considered in these patients, but it should be borne in mind that there is high quality evidence of harm in patients with spontaneous intracerebral haemorrhage. If platelet transfusion is required, the recommended dose is 2–3 adult doses (units).
Restarting anticoagulation
Registry studies have demonstrated a benefit for restarting anticoagulation in the warfarin era following both spontaneous and traumatic intracranial haemorrhage, and gastrointestinal haemorrhage.50,51 Similar results have been found with DOACs.52
On-going, randomised, prospective trials aim to confirm the observations seen in retrospective studies, and elucidate the optimal timing of restarting therapy particularly for intracranial haemorrhage.53
Practically, the evidence of benefit is fairly convincing, but decisions need to be made on a case-by-case basis with the involvement of the patient, their family, and an expert multidisciplinary team. At the very least, patients should be discharged with a robust plan for timely expert review of the appropriateness of anticoagulation usually with haematology.
close window and return to take test
References
1. Hoffmann TC, Mar C Del. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2017;177:407–19. https://doi.org/10.1001/jamainternmed.2016.8254
2. Herrera-Perez D, Haslam A, Crain T, et al. Meta-research: a comprehensive review of randomized clinical trials in three medical journals reveals 396 medical reversals. Elife 2019;8:1–19. https://doi.org/10.7554/eLife.45183.001
3. Baharoglu MI, Cordonnier C, Salman RAS, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016;387:2605–13. https://doi.org/10.1016/S0140-6736(16)30392-0
4. Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202–04. https://doi.org/10.1111/j.1538-7836.2009.03678.x
5. Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomised trial. Ann Intern Med 2009;150:293–300. https://doi.org/10.7326/0003-4819-150-5-200903030-00005
6. Garcia DA, Regan S, Crowther M, Hylek EM. The risk of hemorrhage among patients with warfarin-associated coagulopathy. J Am Coll Cardiol 2006;47:804–08. https://doi.org/10.1016/j.jacc.2005.09.058
7. Parry-Jones AR, Di Napoli M, Goldstein JN, et al. Reversal strategies for vitamin K antagonists in acute intracerebral hemorrhage. Ann Neurol 2015;78:54–62. https://doi.org/10.1002/ana.24416
8. Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013;128:1234–43. https://doi.org/10.1161/CIRCULATIONAHA.113.002283
9. Chai-Adisaksopha C, Hillis C, Siegal DM, et al. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal: A systematic review and meta-analysis. Thromb Haemost 2016;116:879–890. https://doi.org/10.1160/TH16-04-0266
10. Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus 2010;8:149–54. https://doi.org/10.2450/2010.0149-09
11. Lubetsky A, Yonath H, Olchovsky D, et al. Comparison of oral vs Intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med 2003;163:2469–73. https://doi.org/10.1001/archinte.163.20.2469
12. Electronic Medicines Compendium (emc). Beriplex P/N 250 IU. Summary of Product Characteristics (SmPC). https://www.medicines.org.uk/emc/product/6354
13. Electronic Medicines Compendium (emc). Octaplex 500 IU powder and solvent for solution for injection – Summary of Product Characteristics (SmPC). https://www.medicines.org.uk/emc/product/6566/smpc#gref [accessed 21 Feb 2021].
14. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost 2018;16:209–19. https://doi.org/10.1111/jth.13912
15. Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of ADAMTS13. Int J Lab Hematol 2020;42:685–96. https://doi.org/10.1055/s-0038-1627480
16. Patel JP, Byrne RA, Patel RK, Arya R. Progress in the monitoring of direct oral anticoagulant therapy. Br J Haematol 2019;184:912–24. https://doi.org/10.1111/bjh.15756
17. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of dactor Xa inhibitor activity. N Engl J Med 2015;373:2413–24. https://doi.org/10.1056/NEJMoa1510991
18. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019;380:1326–35. https://doi.org/10.1056/NEJMoa1814051
19. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9. https://doi.org/10.1161/CIRCULATIONAHA.111.029017
20. Levi M, Moore KT, Castillejos CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost 2014;12:1428–36. https://doi.org/10.1111/jth.12599
21. Levy JH, Moore KT, Neal MD, et al. Rivaroxaban reversal with prothrombin complex concentrate or tranexamic acid in healthy volunteers. J Thromb Haemost 2018;16:54–64. https://doi.org/10.1111/jth.13894
22. Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation 2015;131:82–90. https://doi.org/10.1161/CIRCULATIONAHA.114.013445
23. Friedberg JW. How I treat double-hit lymphoma. Blood 2017;130:590–6. https://doi.org/10.1182/blood-2017-04-737320
24. Lu G, Pine P, DeGuzman F, et al. Reversal of anticoagulation effects of rivaroxaban and associated bleeding in a rabbit acute hemorrhage model by andexanet alfa vs. coagulation replacement factors (P5.054). Neurology 2017;88(16 Supplement P5.054):1526-1632.
25. Lu G, Pine P, Leeds JM, et al. Andexanet alfa effectively reverses edoxaban anticoagulation effects and associated bleeding in a rabbit acute hemorrhage model. PLoS One 2018;13:e0195122. https://doi.org/10.1371/journal.pone.0195122
26. Grottke O, Braunschweig T, Rossaint R, et al. Transient or extended reversal of apixaban anticoagulation by andexanet alfa is equally effective in a porcine polytrauma model. Br J Anaesth 2019;123:86–95. https://doi.org/10.1016/j.bja.2019.04.059
27. Gómez-Outes A, Alcubilla P, Calvo-Rojas G, et al. Meta-analysis of reversal agents for severe bleeding associated with direct oral anticoagulants. J Am Coll Cardiol 2021;77:2987–3001. https://doi.org/10.1016/j.jacc.2021.04.061
28. Green L, Tan J, Morris JK, et al. A three-year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (Orange study). Haematologica 2018;103:738–45. https://doi.org/10.3324/haematol.2017.182220
29. Cohen AT, Lewis M, Connor A, et al. 30 day mortality following andexanet alfa in ANNEXA-4 compared with prothrombin complex concentrate (PCC) therapy in the ORANGE study for life-threatening non-vitamin K oral anticoagulant (NOAC) related bleeding. J Am Coll Cardiol 2020;75:2242. https://doi.org/10.1016/S0735-1097(20)32869-2
30. ClinicalTrials.gov. Trial of Andexanet in ICH Patients Receiving an Oral FXa Inhibitor. https://clinicaltrials.gov/ct2/show/NCT03661528 [last accessed 27 August 2021).
31. Pollack C V., Reilly PA, Van Ryn J, et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med 2017;377:431–41. https://doi.org/10.1056/NEJMoa1707278
32. Milling TJ, Pollack C V. A review of guidelines on anticoagulation reversal across different clinical scenarios – is there a general consensus? Am J Emerg Med 2020;38:1890–1903. https://doi.org/10.1016/j.ajem.2020.05.086
33. National Institute for Health and Care Excellence. Andexanet alfa for reversing anticoagulation from apixaban and rivaroxaban.Technology Appraisal Guidance [TA697]. Published 12th May 2021. https://www.nice.org.uk/guidance/TA697 [last accessed 26th August 2021].
34. Scottish Medicines Consortium. Andexanet alfa (Ondexxya). 2020. https://www.scottishmedicines.org.uk/medicines-advice/andexanet-alfa-ondexxya-full-smc2273/ [last accessed 13 October 2020].
35. Electronic Medicines Compendium (emc). Ondexxya 200 mg powder for solution for infusion. Summary of Product Characteristics (SmPC). https://www.medicines.org.uk/emc/product/10933/smpc#gref [last accessed 27th August 2021].
36. Electronic Medicines Compendium (emc). Praxbind 2.5 g/50 mL solution for injection/infusion. Summary of Product Characteristics (SmPC). https://www.medicines.org.uk/emc/medicine/31243#gref [last accessed 27th August 2021].
37. UpToDate. Direct oral anticoagulant reversal agents for life-threatening bleeding. https://www.uptodate.com/contents/image/print?imageKey=HEME%2F112299 [last accessed 27th August 2021).
38. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–27. https://doi.org/10.1160/TH09-11-0758
39. Ollier E, Hodin S, Lanoiselée J, et al. Effect of activated charcoal on rivaroxaban complex absorption. Clin Pharmacokinet 2017;56:793–801. https://doi.org/10.1007/s40262-016-0485-1
40. Wang X, Mondal S, Wang J, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs 2014;14:147. https://doi.org/10.1007/s40256-013-0055-y
41. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018;154:1121–1201. https://doi.org/10.1016/j.chest.2018.07.040
42. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:594–622. https://doi.org/10.1016/j.jacc.2020.04.053
43. Makris M, Van Veen JJ, Tait CR, Mumford AD, Laffan M. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol 2013;160:35–46. https://doi.org/10.1111/bjh.12107
44. Electronic Medicines Compendium (emc). Prosulf 10 mg/ml Solution for Injection, Summary of Product Characteristics (SmPC). https://www.medicines.org.uk/emc/product/8#gref [last accessed 19th June 2021].
45. Schwartz HJ, Aaronson DW, Beck SA. Summary statement. Protamine. J Allergy Clin Immunol 1998;101:S507–S509.
46. Adcock DM, Gosselin RC. The danger of relying on the APTT and PT in patients on DOAC therapy, a potential patient safety issue. Int J Lab Hematol 2017;39:37–40. https://doi.org/10.1111/ijlh.12658
47. FDA, PFIZER. FRAGMIN® (dalteparin sodium) injection, for subcutaneous use. Initial U.S. Approval:1994.1998;50: 1–25.
48. Bijsterveld NR, Arno MH, Boekholdt SM, et al. Ability of recombinant factor VIIa to reverse the anticoagulant effect of the pentasaccharide fondaparinux in healthy volunteers. Circulation 2002;106:2550–4. https://doi.org/10.1161/01.cir.0000038501.87442.02
49. Corbonnois G, Martin M, Hacquard M, et al. Fondaparinux reversal with activated prothrombin complex concentrate in anesthetised bleeding rats. Thromb Haemost 2013;109:560–3. https://doi.org/10.1160/TH12-08-0575
50. Staerk L, Lip GYH, Olesen JB, et al. Stroke and recurrent haemorrhage associated with antithrombotic treatment after gastrointestinal bleeding in patients with atrial fibrillation: Nationwide cohort STUDY. BMJ 2015;351:h5876. https://doi.org/10.1136/bmj.h5876
51. Nielsen PB, Larsen TB, Skjøth F, et al. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A Nationwide cohort study. Circulation 2015;132:517–25. https://doi.org/10.1161/CIRCULATIONAHA.115.015735
52. Zhou Y, Guo Y, Liu D, Feng H, Liu J. Restarting of anticoagulation in patients with atrial fibrillation after major bleeding: a meta-analysis. J Clin Pharm Ther 2020;45:591–601. https://doi.org/10.1111/jcpt.13130
53. King B, Milling T, Gajewski B, et al. Restarting and timing of oral anticoagulation after traumatic intracranial hemorrhage: A review and summary of ongoing and planned prospective randomized clinical trials. Trauma Surg Acute Care Open 2020;5:e000605. https://doi.org/10.1136/tsaco-2020-000605

All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.