Digoxin is widely used in the management of atrial fibrillation (AF) and the heart failure (HF) syndrome. Significant numbers of people are prescribed digoxin every year; 2,560,941 prescriptions for digoxin were issued by general practitioners in England in 2023.1 Digoxin predominantly acts by increasing vagal tone. This reduces heart rate and increases diastolic filling of the left ventricle (LV), resulting in an improvement in LV function. Coronary artery perfusion, which predominantly occurs during diastole, also increases. Digoxin increases intracellular Ca2+ concentrations as a result of inhibition of the Na+/K+ adenosine triphosphatase, and so myocardial contractility increases. However, it has limitations, which include a narrow therapeutic index, and interactions with drugs with which it is commonly co-prescribed. Moreover, it can cause problematic side effects. In acute overdose, or acute-on-chronic accumulation causing toxicity, digoxin presents some unique challenges – we will briefly consider some of these issues here.
Laboratory testing of digoxin levels
Laboratories in the National Health Service (NHS) acute trusts will have arrangements in place for routine therapeutic drug level monitoring of digoxin. Routine assays may not be available on a daily basis. However, local arrangements should exist to ensure the availability of urgent testing in cases of suspected digoxin toxicity 24 hours a day.
One commonly used method is the electro-chemiluminescence immunoassay (Roche ECLIA®). This assay involves adding a combination of Ruthenium-labelled digoxin-specific monoclonal antibodies, a digoxin derivative labelled with biotin, and a fluorescent marker attached to streptavidin to the sample. Biotin and streptavidin form an extremely strong non-covalent chemical bond. Ruthenium is included due to its unique magnetic properties. The mixed sample is then incubated to ensure binding of the antibodies to the digoxin in the sample. The complexes formed are captured magnetically and a current passed across these, which then fluoresce. This allows automated calculation of the digoxin concentration in the sample, derived from the change in fluorescence measured against a control sample.
Each assay will take around one hour to process, typically made up of:
- Calibration: 20 minutes (not required for every test)
- Assay: 18–20 minutes
- Quality assurance processes: 20 minutes.
Therefore, even if calibration of the assay is required, a result should still be available within a turnaround time of approximately one hour, subject to local quality-assurance processes and reporting arrangements.
Assay interference can occur when drugs with structural similarities to digoxin are co-prescribed. This used to be especially common with spironolactone. However, through iterative design improvements, the assays available today have largely overcome this problem and spironolactone interference is not clinically significant. The Roche Eclia® assay outlined above has eliminated this problem entirely.
Digoxin-like compounds, such as those found in European Yew (Taxus baccata) and the bufadienolides found in most toads (genus Bufo), and more of toxicological interest, are very rarely encountered in clinical practice in the UK and will not be considered here.
At very high digoxin levels (>6.5 µg/L), commercial assays start to lose their accuracy and may become less reliable. The laboratory will usually identify this phenomenon, if it occurs, and undertake further testing. However, it is important that the laboratory is satisfied with the result issued if faced with an extremely high digoxin level, as having an accurate level will assist clinicians to determine appropriate management.
Pharmacokinetics of digoxin
Digoxin has some pharmacokinetic features which are particularly important when considering toxicity. Approximately 75% of an administered dose of digoxin is excreted via the kidneys, with the other 25% excreted via the biliary tract. It has an elimination half-life of 36–48 hours.2 With a decline in renal function, the half-life of digoxin increases, causing serum levels to rise. This may cause a therapeutic level to become a potentially toxic level within a short time, especially if other problems such as dehydration or electrolyte abnormalities, such as hypokalaemia, are present.
Hypokalaemia is a significant risk factor for developing toxicity as digoxin competes with K+ for binding sites on the ɑ-subunit of the Na+/K+-adenosine triphosphatase (ATPase) pump. Hypokalaemia results in more available binding sites on the Na+/K+-ATPase pump to which digoxin can bind.3 Many patients on digoxin will also be on diuretics, the majority of which increase the risk of developing hypokalaemia and impaired renal function, potentially resulting in toxicity.
An important issue is that of the narrow therapeutic index which digoxin exhibits. Changes to medications – the addition of an interacting drug, such as spironolactone, or modest changes in renal function – can dramatically alter the pharmacokinetic profile of digoxin and lead to toxicity, even at modest doses. The therapeutic range for digoxin is generally accepted to be around 0.8–2.0 µg/L. However, it is not widely recognised that significant toxicity can exist in a patient with a digoxin level within an apparently therapeutic range. This is a particular problem in the elderly. Age-related changes in organs such as heart, kidneys and gut render the older patient less able to eliminate digoxin efficiently and at increased risk of side effects and toxicity.
Side effects of digoxin
Side effects of digoxin are common and include visual disturbances, particularly blurring of vision. This is due to inhibition of Na+/K+-ATPase in retinal cone cells which express the ɑ-subunit proteins to which digoxin binds. Xanthopsia, a perception of a yellowish tinge to perceived light or yellow halos around lights, is relatively uncommon but well recognised.
Other side effects include confusion, gastrointestinal problems (especially diarrhoea), bradycardia, hypotension, and supraventricular tachycardia. Each of these is mediated by blockade of Na+/K+-ATPase. In the case of diarrhoea, blockade of Na+/K+-ATPase on the luminal gut surface reduces water reabsorption and leads to excess water accumulating in the gut lumen.
These side effects might be diminished or abolished with a reduction in digoxin dose, but some patients will develop side effects, even at doses as low as 62.5 µg/day. For those patients for whom side effects are intolerable, the only option is to stop the digoxin and prescribe an alternative medication.
All of the commonly observed side effects of digoxin stem from the inhibition of Na+/K+-ATPase, and it is this ability to inhibit Na+/K+-ATPase that makes digoxin a useful addition to the formulary – and especially dangerous in toxicity.
Digoxin toxicity
A common misconception is that the classic ‘reverse-tick’ appearance of the ST-segment of the electrocardiogram (ECG) is diagnostic of toxicity. Its presence does not confirm toxicity but is rather a well-characterised effect on the ECG of digoxin use. It is a common finding in the ECG of many asymptomatic patients on digoxin at therapeutic doses and who have therapeutic plasma levels. The characteristic appearance of the ECG with this so-called ‘digoxin effect’ is shown in figure 1.
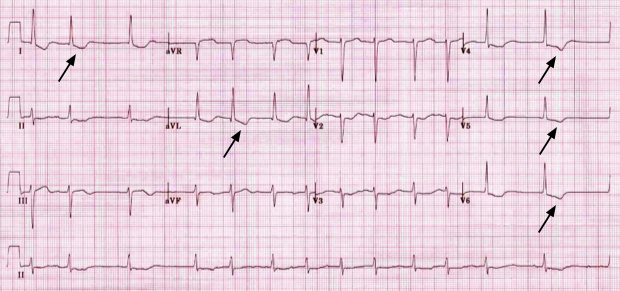
| Dr CA Blakemore, FRCP. Personal collection |
Digoxin toxicity with clinical signs, symptoms and a cardiac rhythm disturbance is an important medical emergency. It is potentially life threatening and because the majority of cases of toxicity arise as a result of chronic accumulation of digoxin – most commonly in the setting of deteriorating chronic kidney disease rather than chronic deliberate excess or acute overdose – the signs and symptoms are easily missed. It is difficult to be certain of the mortality rate associated with digoxin toxicity, but Peters et al. found that in 727 digoxin-toxic patients treated over a 20-year period, the inpatient mortality and mortality at 1 year was estimated to be 12.7% and 42.7%, respectively.4 In a non-interventional, observational UK registry study of 87 patients with digoxin toxicity who were treated with Digifab®, Thomas et al. found that three patients died from digoxin poisoning during the period surveyed. This suggested a mortality rate for patients with clinical features of toxicity, who were given DIGIFab®, of 3.4%.5
Many patients on digoxin also take other medications, which act through various mechanisms which have been well described.6,7 Examples include:
- warfarin, which inhibits protein-binding of digoxin, thus increasing its free drug ratio
- amiodarone, which increases serum concentrations of digoxin by inhibiting renal excretion and P-glycoprotein-mediated intestinal export of digoxin
- spironolactone, which inhibits renal tubular secretion of digoxin
- beta blockers and heart rate-reducing calcium-channel blockers (benzothiazepine – diltiazem and phenylalkylamine – verapamil) potentiate the heart rate-lowering effect of digoxin and, if required, should be commenced cautiously while monitoring the patient for signs of toxicity.
Commonly seen features of digoxin toxicity include:
- vomiting
- diarrhoea
- confusion
- blurred vision
- brady- or tachy-arrhythmias
- hypotension
- all degrees of heart block
- seizures (an ominous sign)
- cardiac arrest.
Management of digoxin toxicity
There is a potential risk in correcting hypocalcaemia as in digoxin toxicity, free Ca2+ binds to cardiac troponin C, causing irreversible systolic arrest or ‘stone-heart syndrome’ in extreme cases. It is so rarely reported that we regard it as an almost hypothetical risk, but nevertheless, care must be taken if replacing Ca2+ in a patient with digoxin toxicity, as intracellular Ca2+ levels are already high. The combination of hyperkalaemia and hypocalcaemia in a patient with features of digoxin toxicity would suggest that a very significant Na+/K+-ATPase blockade has occurred and that the patient is potentially critically unwell.
Managing arrythmias
Bradycardias
Bradycardia without adverse features generally requires no specific treatment. Digoxin should be withheld, and the patient monitored for signs of serious toxicity. Where significant bradycardia or heart block exists with hypotension, atropine at a dose of 0.5–1 mg (repeated to a maximum of 3 mg) can be effective and should be considered.
Ventricular tachycardias
- Direct current (DC) cardioversion
Bradycardia-associated or pause-dependent ventricular tachycardia (VT) is an emergency which requires immediate management. DC cardioversion in the setting of digoxin toxicity presents significant risks of mortality, even using defibrillators at low-energy settings. For this reason, it is best avoided, unless the circumstances are such that no alternative is available. In the event of cardiac arrest, standard advanced life support should be commenced immediately, and expert advice sought.
A caveat applies to the use of isoprenaline for bradycardia with hypotension, where accurately titrating the dose of isoprenaline to increase heart rate and blood pressure, whilst minimising the risk of VT, can be problematic.
- Cardiac pacing
Cardiac pacing has a role to play in limited circumstances. In the setting of extreme bradycardia or a high-degree atrioventricular block, temporary pacing can achieve control of the heart rate, which will usually also improve blood pressure. With bradycardia-associated VT, the substrate for the VT is the profound bradycardia, and pacing will usually abolish the VT. However, even experienced clinicians are sometimes unaware that the myocardium can be both excitable and refractory to pacing. Consequently, a pacing potential of 1V or more may be required to obtain electromechanical capture. The act of passing a pacing wire across the tricuspid valve and into the right ventricle can precipitate asystole, VT or ventricular fibrillation (VF).
- Transcutaneous pacing
Transcutaneous pacing via an external defibrillator can also be effective as a bridge to definitive treatment, but similar issues arise. It is often forgotten that the patient may suffer significant discomfort as a result of the delivery of electrical energy via the skin surface whilst they are conscious. It is important to consider administering an intravenous sedative – such as a benzodiazepine – to the patient if this option is to be used. A significant risk is that of obtaining electrical capture and neglecting to confirm that mechanical capture has been achieved.
Reducing digoxin levels
Extracorporeal elimination of digoxin
Digoxin has an apparent volume of distribution of approximately 5–10 L/kg.8 This, coupled with its protein binding of around 20%, renders extracorporeal elimination unhelpful. This is due to the minimal clearance of digoxin from circulating plasma. The fact that many patients are on digoxin for the heart failure (HF) syndrome also makes it an unappealing treatment – although flow rates through the peristaltic pump of a dialysis machine are infinitely adjustable, effective dialysis or haemofiltration requires rapid rates of blood flow. A blood flow of approximately 300–500 ml/min is required for effective dialysis, which can cause significant myocardial strain. This makes extracorporeal elimination ineffective and, in patients with the HF syndrome, potentially unsafe. Peritoneal dialysis (PD) does not cause the same degree of myocardial strain as haemodialysis or hemofiltration, but it is ineffective, as the clearance of digoxin via this method is minimal.
Digoxin immune antibody fragments (fab)
Digoxin immune fab (DIGIFab®) is an ovine digoxin-specific antibody which acts as an antidote by binding to digoxin with greater affinity than digoxin binds to the cellular membrane Na+/K+-ATPase. It is excreted via the kidneys, with an elimination half-life of approximately 20 hours.8
Importantly, the digoxin-fab complexes will begin to dissociate after approximately 12 hours, and further doses may be required if signs of toxicity recur.9 The decision to give repeat doses should be based entirely on the features of toxicity. Repeat doses should not be given routinely.
Box 1. Indications for the use of DIGIFab® in suspected/confirmed digoxin toxicity11
| Ventricular tachycardia |
| Ventricular fibrillation |
| Asystole |
| Symptomatic high-degree atrioventricular block |
| Serum K+ >6.5 mmol/L |
| Hypotension associated with end-organ dysfunction |
Generally accepted indications for the use of DIGIFab® are listed in box 1. According to NICE, digoxin-specific antibody (DIGIFab®) is indicated in the
‘treatment of known or strongly suspected life-threatening digoxin toxicity associated with ventricular arrhythmias or bradyarrhythmias unresponsive to atropine and when measures beyond the withdrawal of digoxin and correction of any electrolyte abnormalities are considered necessary.’10
DIGIFab® is expensive, costing approximately £750 per 40 mg vial. It is unusual to need fewer than three vials in the setting of significant digoxin toxicity and as many as five vials may need to be given to those patients presenting with life-threatening toxicity. For administration, each vial of DIGIFab® powder is dissolved in 4 ml of water for injections, taking care to ensure full dissolution. The total dose to be administered can then be injected into an appropriate volume of 0.9% saline (typically 100 ml) and administered over 30–60 minutes.
Dosing of DIGIFab® can be complex. Patients with toxicity tend to present or run into difficulty late at night when vagal tone is at its greatest. In most hospitals in the NHS, it is likely that fewer, less-experienced clinical staff will be on duty at night compared with daytime levels of cover.
The following rule of thumb can be used to determine the number of vials needed to neutralise half the circulating levels of digoxin:
Number of 40mg vials of DIGIFab® needed is equal to:
a) Ingested dose (in mg) × 0.8
OR
b) Serum digoxin level (in ng/ml or µg/L) × weight (in kg) × 0.005
In either case, the dose can be rounded to the nearest vial. Doses and, therefore, costs of DIGIFab® for equimolar neutralisation of circulating digoxin may be very high; in most clinical scenarios, it is not necessary to offer full neutralisation of digoxin.8
There are no formal guidelines for the management of digoxin toxicity, but a recent consensus document suggested administering five vials of DIGIFab® in the setting of life-threatening toxicity.11 However, the majority of patients with digoxin toxicity can be managed conservatively without the need for DIGIFab®. In mild cases, stopping digoxin might be all that is required. For those patients with more serious – or life-threatening – toxicity, DIGIFab® can be very effective.

Case study
An 82-year-old male patient, with a past medical history of atrial fibrillation (HF), mitral regurgitation, HF and chronic kidney disease stage 3, presented with breathlessness and episodes of collapse with loss of consciousness lasting a few seconds. He recovered quickly in between episodes, without confusion or drowsiness. His medications and observations upon admission are shown in table 1.
Table 1. Case study observations and medications
| Observations | Medications | ||
| Parameter | Result | Name | Dose |
| Temperature | 36.8°C | Edoxaban | 60 mg OD |
| Pulse rate | 29 bpm | Furosemide | 40 mg BD |
| Respiratory rate | 16 breaths/min | Ramipril | 2.5 mg OD |
| Blood pressure | 95/50 mmHg | Digoxin | 187.5 μg OD |
| Oxygen saturations | 100% on 15 L O2 (via reservoir mask) | Spironolactone | 12.5 mg OD |
| Key: BD = twice daily; bpm = beats per minute; OD = once daily | |||
Multiple ECG rhythm strips were provided by the paramedic crew. These showed AF with a slow ventricular response (20–30 beats per minute [bpm]), with occasional episodes of pauses lasting 5–10 seconds. The crew reported that these episodes had occurred approximately every five minutes, and they had captured two episodes of pauses on the rhythm strips.
Bloods were taken. The results are shown in table 2.
Table 2. Case study blood test results
| Parameter | Result (reference range) |
| Sodium | 132 mmol/L (133–146 mmol/L) |
| Potassium | 5.8 mmol/L (3.5–5.3 mmol/L) |
| Urea | 12 mmol/L (2.5–7.8 mmol/L) |
| Creatinine | 250 μmol/L (59–104 μmol/L) |
| Digoxin level (6-hr post-dose) | 4.4 μg/L (0.8–2 μg/L) |
His ECG showed AF with a ventricular rate of 22 bpm. He had an episode of unresponsiveness lasting 7 seconds, which corresponded with a long pause seen on a cardiac monitor. The decision was taken to prescribe and administer four vials of DIGIFab®.
Whilst the DIGIFab® was being obtained from the pharmacy, he had several longer pauses and then a cardiac arrest for which he received advanced cardiopulmonary resuscitation, which successfully restored circulation after eight minutes. All four vials of DIGIFab® were administered and after twenty minutes, the ECG had started to normalise, there were no further episodes of asystole or pauses, and following a two-day stay in the intensive care unit, he was transferred to a cardiology ward and discharged home after a further four-day stay. He did not require any further DIGIFab® after the 4-vial dose administered in the emergency department.
Conclusion
The management of digoxin toxicity is complex; we have outlined a few of the difficulties it can present here. Its affinity for the Na+/K+-ATPase is a powerful tool in the management of patients with cardiac failure or atrial fibrillation; it is also the feature which makes it a dangerous challenge to overcome in severe toxicity.
The most important message is that the management of digoxin toxicity depends on simple things done well – attention to electrolyte imbalance, an appreciation of the effects of other drugs the patient may be taking, and the use of DIGIFab® where appropriate. Paradoxically, it is important to remember that some of the more advanced measures often used in patients with bradycardia may sometimes cause harm.
Key messages
- Digoxin is widely used and has a narrow therapeutic index. It is potentially dangerous if allowed to accumulate, most commonly due to deterioration in kidney function. It is important to remember that many drugs commonly co-prescribed with digoxin can increase the risk of toxicity
- Digoxin levels are measured in plasma – where it is inactive – rather than at the tissue-binding sites, which are central to its toxicity
- Hypokalaemia is a significant risk factor for digoxin toxicity and should be recognised and treated in patients on digoxin
- Injudicious use of isoprenaline, cardiac pacing and direct current cardioversion may simply replace one set of difficulties with another, and so attention must be paid to the risks and benefits of these treatments. When pacing, using either transvenous or transcutaneous techniques, it is vital to ensure that mechanical, as well as electrical capture, has been achieved
- DIGIFab®, although expensive, appears to be effective, safe, easy to administer and well tolerated in most patients. It should not be the first-line option for all patients. However, for those with significant toxicity, it is indicated and probably cost-effective. We are not convinced by arguments relating to reduction in length of hospital stay and reduced need for critical care input for those treated with DIGIFab®, as each case turns on its individual facts and the management of digoxin toxicity should never be an actuarial exercise.
Articles in this supplement
Digoxin: a look back and a look forward
Digitalis – from Withering to the 21st century
The modern-day role of digoxin in heart failure and atrial fibrillation – benefits and limitations
Conflicts of interest
Both authors received an honorarium for their work on this supplement.
Paul J Andrews
Consultant Physician
Harvey Thompson
Foundation Year One Doctor
Torbay Hospital, Torbay and South Devon NHS Foundation Trust, Torquay, Devon
References
1. OpenPrescribing.net. 2024. Available at: https://openprescribing.net/chemical/0201010F0/ (accessed 01 April 2024)
2. Patocka J, Nepovimova E, Wu W, Kuca K. Digoxin: pharmacology and toxicology – a review. Environ Toxicol Pharmacol 2020;79:103400. https://doi.org/10.1016/j.etap.2020.103400 [Epub online ahead of print]
3. Chan KE, Lazarus JM, Hakim RM. Digoxin associates with mortality in ESRD. J Am Soc Nephrol 2010;21:1550–9. https://doi.org/10.1681/ASN.2009101047 [Epub online ahead of print]
4. Peters AE, Chiswell K, Hofmann P, Ambrosy A, Fudim M. Characteristics and outcomes of suspected digoxin toxicity and immune Fab treatment over the past two decades – 2000–2020. Am J Cardiol 2022;183:129–36. https://doi.org/10.1016/j.amjcard.2022.08.004 [Epub online ahead of print]
5. Thomas E, Tomlinson S, Thomas S et al. Treatment of life-threatening digoxin toxicity with digoxin-specific antibody fragments: results from a prospective, non-interventional observational UK patient registry study. Eur J Hosp Pharm 2023;30:e34. https://doi.org/10.1136/ejhpharm-2022-003416 [Epub online ahead of print]
6. Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol 2014;63:2176. https://doi.org/10.1016/j.jacc.2013.02.058 (Epub online ahead of print]
7. Rodin SM, Johnson BF. Pharmacokinetic interactions with digoxin. Clin Pharmokinet 1988;15:227–44. https://doi.org/10.2165/00003088-198815040-00003
8. Chan BSH, Buckley NA. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. Clin Toxicol (Phila) 2014;52:824–36. https://doi.org/10.3109/15563650.2014.943907 [Epub online ahead of print]
9. Hassan SA, Goyal A. Digoxin immune fab. Updated 28 Nov 2022. StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK556101/ (accessed 01 April 2024)
10. National Institute of Health and Care Excellence. British National Formulary. Digoxin-specific antibody. Available at: https://bnf.nice.org.uk/drugs/digoxin-specific-antibody/ (accessed 12 June 2024)
11. Andrews P, Anseeuw K, Kotecha D, Lapostolle F, Thanacoody R. Diagnosis and practical management of digoxin toxicity: a narrative review and consensus. Eur J Emerg Med 2023;30:395–401. https://doi.org/10.1097/MEJ.0000000000001065 [Epub online ahead of print]
