A raised level of lipoprotein(a) (Lp[a]) is gaining recognition worldwide as a significant and prevalent risk factor that can predispose affected individuals to premature and progressive cardiovascular disease (CVD). However, challenges remain with regards to the identification of patients with raised Lp(a) levels, limited treatment options and a lack of prospective evidence to support the therapeutic reduction of Lp(a) levels to prevent CVD. We hereby present real-world examples of patients with elevated Lp(a) concentrations as the underlying aetiology for atherosclerotic cardiovascular disease (ASCVD), illustrating the key principles of diagnosis and management to date.
Background
A vast amount of epidemiological evidence now points towards lipoprotein(a) (Lp[a]) as a strong, genetically determined independent risk factor for cardiovascular disease (CVD).1 Despite this growing body of evidence, challenges and barriers remain globally in the identification of patients with raised Lp(a) levels. The European Atherosclerosis Society (EAS) Lipid Clinics Network conducted a survey involving a total of 151 centres to identify and understand how and when Lp(a) is tested and clinically evaluated in lipid clinics throughout Europe, and the challenges that may prevent evaluation.2 The survey found that 75.5% of clinicians routinely measure Lp(a) in clinical practice. The most common reasons for not measuring Lp(a) were the lack of reimbursement or of treatment options, the local non-availability of an Lp(a) test and the high cost of performing the laboratory test.2 Clinicians declared that availability of specific therapies to reduce Lp(a) levels would result in a greater commitment to Lp(a) testing.
Amongst those who routinely measure Lp(a), the measurement is often utilised to further stratify patients’ cardiovascular (CV) risk.2 Regarding the type of patients considered eligible for Lp(a) testing, 84.2% reported that they would measure Lp(a) in patients with a diagnosis of familial hypercholesterolemia (FH), 78.9% measure Lp(a) in patients with recurrent CV events – despite low-density lipoprotein cholesterol (LDL-C) lowering – and 68.4% measure Lp(a) at least once in each adult person’s lifetime.2
There is also an inherent challenge in communicating a finding of a raised Lp(a) level to patients, considering the lack of licensed treatment options to reduce Lp(a); care is needed to avoid provoking anxiety or discouragement. Management of other CV risk factors, lifestyle changes and strategies to manage anxiety may all form part of a discussion with a patient regarding their raised Lp(a) level.
We hereby present several real-world cases of patients with elevated Lp(a) levels as the underlying aetiology for atherosclerotic cardiovascular disease (ASCVD), highlighting the importance of screening for Lp(a) amongst high-risk individuals and of communicating the associated CV risks to these patients. These cases also serve to highlight current challenges that clinicians face in the diagnosis and management of raised Lp(a) concentrations, which ongoing prospective research may help to resolve in future.
The following cases represent patients who were seen at a tertiary care lipid management clinic; not all treatment options may be available to cardiologists working in other hospital settings.
Case 1
Treatment of raised Lp(a) levels in refractory angina
A 42-year-old gentleman presented to our centre with unstable angina. He had no previous cardiac history, had a balanced diet and had never smoked. Coronary angiography revealed diffuse, multi-vessel coronary artery disease (CAD). Subsequently, in the context of ongoing angina he was treated with quadruple coronary artery bypass graft (CABG) surgery and a total of 12 stents over a three-year period. He averaged a new coronary stent every four to six weeks. During this period, there was no evidence of myocardial infarction (MI); however, there was severe and dynamic progression of the native CAD and venous grafts.
Despite these multiple and extensive coronary interventions and being treated with optimal medical therapy throughout, he continued to experience ongoing angina which was adversely affecting his quality of life. At this point, his Lp(a) level was measured and was found to be significantly elevated at 1,200 mg/L (normal range: 0–300 mg/L). The remainder of his fasting lipid profile revealed normal total cholesterol (TC) (3.2 mmol/L; normal range <5 mmol/L), low-density lipoprotein cholesterol (LDL-C) (1.5 mmol/L; normal range <3 mmol/L) and plasma triglycerides (TG) (1.4 mmol/L; normal range <1.7 mmol/L).
The patient was advised that lipoprotein apheresis (LA) is currently the most effective available means of reducing Lp(a) levels. After a discussion regarding the quality-of-life impact of LA treatment (with no alternative Lp(a)-specific therapeutic options available) versus the current negative effects of his ongoing angina, the patient agreed to begin a fortnightly regimen of LA using the Kaneka whole blood system (DX21, Kaneka Medical Products, Osaka, Japan). LA significantly reduced the plasma levels of LDL-C, TC, TG and most importantly, Lp(a). Figure 1 demonstrates his Lp(a) levels pre- and post-treatment.
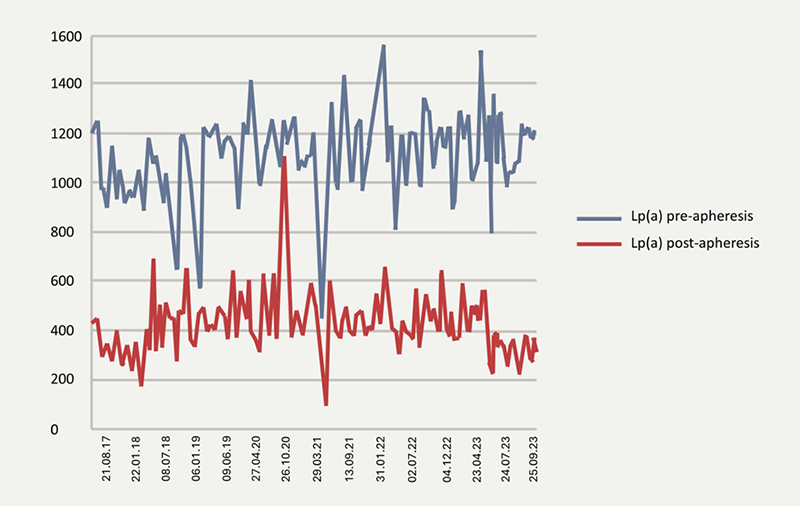
Since the institution of regular LA, the patient has shown improvement in his functional status, with significant improvement of his angina-related chest pain and quality of life. The patient is now active, able to walk unrestricted and is engaged in fulltime employment. LA has also slowed the rate of progression of his CAD evidenced by the reduction in the rate of revascularisation procedures since the institution of LA. During the six-year period in which the patient has been undergoing LA, he has had four prophylactic stents to an in-stent stenosis in the distal right coronary artery (RCA), in contrast to the multiple interventions he required during the three years preceding LA.
This case highlights several noteworthy lessons regarding the management of refractory angina in the context of raised lipoprotein(a) levels. Firstly, Lp(a) is an important risk factor for refractory angina and the progression of CAD; Lp(a) measurement is often a missed feature of the biochemical profile of these challenging patients. Secondly, it shows that in patients for whom LA is an appropriate and available treatment option, it represents an effective tool in normalizing Lp(a), which can impact the rate of progression of dynamic CAD and lead to an improvement in angina symptoms. Further studies at the clinical and mechanistic level are needed to validate and understand this phenomenon.
Case 2
Elevated Lp(a) levels associated with an acute thromboembolic MI
We report the investigation and management of a 49-year-old male, with a background of hypertension, alcohol excess and fatty liver disease, who presented acutely with chest pain and an ECG demonstrating an inferior ST-elevation MI. Coronary angiography revealed only mild, non-obstructive CAD; however, cardiac enzyme levels were significantly elevated with a peak troponin of 41,770 ng/L. Computed tomography (CT) angiogram excluded a pulmonary embolism and aortic dissection.
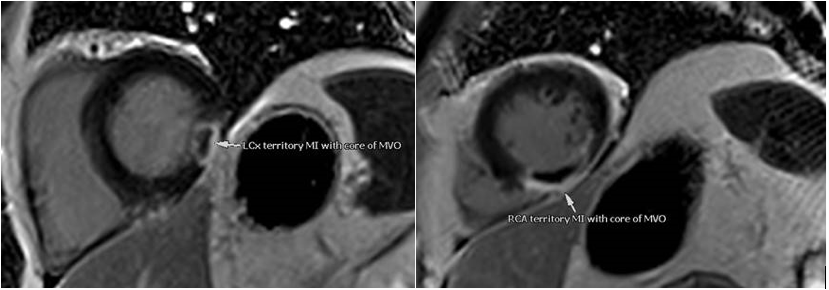
Subsequently, cardiac magnetic resonance imaging (CMR) showed evidence of two large acute transmural infarctions in two separate coronary artery territories involving the left circumflex and right coronary arteries (figure 2), which were deemed secondary to thromboembolism in view of the unobstructed coronary arteries. There was no evidence of a left ventricular thrombus or intra-cardiac shunt on CMR or echocardiography. His fasting lipid profile revealed serum levels of TC of 5.6 mmol/L, LDL-C of 3.30 mmol/L and a significantly elevated Lp(a) level of 322 nmol/L. Haematological investigations, including a thrombophilia screen, have not revealed any underlying pro-coagulation disorders. Hence, the pro-thrombotic effect of his severely raised Lp(a) levels was identified as a contributing risk factor to his presentation with a thromboembolic MI.
He was discharged on anticoagulation with rivaroxaban and single antiplatelet therapy with clopidogrel; statin therapy was introduced to lower his LDL-C. We conducted a detailed discussion with him in which he was advised of his increased Lp(a) level and how this contributed to his risk of further CV events, and the importance of taking steps to reduce his overall risk by managing modifiable risk factors, where possible (such as reducing alcohol intake, following a healthy diet and increasing his physical activity levels). In addition, we advised his general practitioner (GP) of the importance of controlling his additional cardiovascular risk factors such as controlling his hypertension and LDL-C.
Elevated Lp(a) has adverse pro-atherogenic and pro-thrombotic properties and is associated with a higher risk of ASCVD as well as increased risk of thrombosis.1 This case illustrates the role of Lp(a) as a risk factor for acute MI of thromboembolic origin. It highlights the significance of screening Lp(a) levels in patients to identify their risk of CVD and thromboembolic disease and the importance of research into advancement of Lp(a) targeted therapies.
Case 3
Management of raised Lp(a) in FH and ASCVD
Raised Lp(a) levels are more prevalent in heterozygous FH (HeFH) than in the general population (29.3% vs. 22.2%).3 The prevalence of raised Lp(a) levels may be as high as 30–50% amongst patients with HeFH.4 Additionally, patients with homozygous FH (HoFH) due to low-density lipoprotein receptor (LDLR) gene mutations have been reported to have almost twofold higher Lp(a) levels than those with HeFH, signifying a gene-dosage effect.5
A gentleman with HeFH involving the LDLR gene mutation, significantly raised Lp(a) levels and a strong family history of heart disease developed premature CVD; he presented with an acute coronary syndrome at 42 years of age, which required urgent CABG surgery. His only other CV risk factor, aside from dyslipidaemia, was that he was an ex-smoker. He was initially treated with optimised oral lipid-lowering therapy in the form of rosuvastatin 40 mg and ezetimibe 10 mg, which was successful in lowering his LDL-C to 3.1 mmol/L down from a baseline value of 9.3 mmol/L; however, his Lp(a) remained residually raised at 2,020 mg/L (normal range: 0–300 mg/L). Therefore, a combination of 1,000 mg of nicotinic acid and laropiprant 20 mg was added to his therapy. Although this reduced his Lp(a) level by 27% to 1,470 mg/L, it also caused debilitating side effects of skin rash, gastrointestinal complaints and mood changes. This medication was subsequently withdrawn by the European Medicines Agency based on findings from the HPS2-THRIVE trial, which showed that this drug does not reduce major adverse cardiac events and causes a higher incidence of serious non-fatal side effects.6 His treatment with this medication was therefore discontinued.
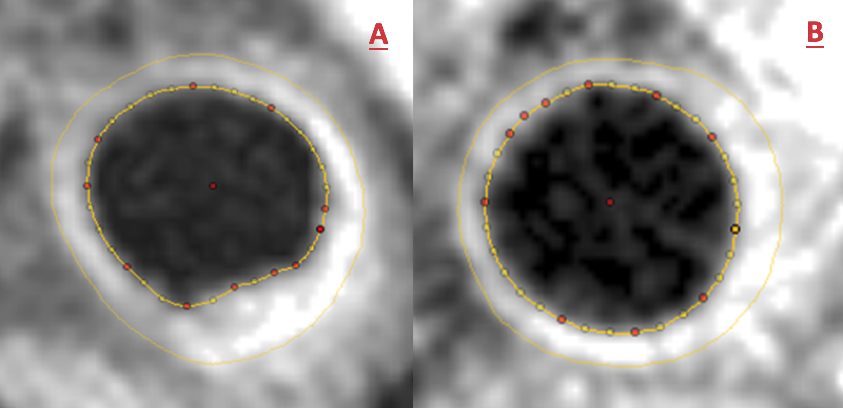
Given that this gentleman was symptomatic with refractory angina affecting his quality of life, he was recruited into a randomised controlled cross-over clinical trial in patients with refractory angina and elevated Lp(a) levels ≥500 mg/L, randomised to three months of weekly LA or sham procedures, followed by cross-over.7 This trial demonstrated that LA resulted in significant improvements in quantitative myocardial perfusion, carotid atheroma burden, exercise capacity, quality of life and symptoms, including angina, with no significant change observed in the sham treatment arm.7 Figure 3 depicts carotid plaque regression observed in this patient during LA, demonstrated with cross-sectional carotid CMR before (figure 3A) and after (figure 3B) three months of LA in the same location of his left common carotid artery.
After the clinical trial was completed, fortnightly LA was continued for this patient on clinical grounds due to his symptomatic benefit, resulting in 80% acute reductions in Lp(a) levels. He achieved a post-LA Lp(a) level of 401 mg/L and LDL-C of 0.98 mmol/L. Subsequently, he was initiated on a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor in the form of evolocumab 140 mg subcutaneously every fortnight for LDL-C reduction in addition to his other treatments. Evolucumab is not licensed for Lp(a) reduction, although there is some evidence that it is associated with modest reductions in Lp(a) levels. Three months after evolocumab treatment was initiated, there had been a further incremental reduction of his Lp(a) level to 353 mg/L and LDL-C level to 0.34 mmol/L with current Lp(a) levels of 120 nmol/L using the current isoform insensitive assay. More importantly, this patient has not required any further revascularisation procedures during the past five years that this combined therapeutic approach has been utilised and remains completely free of the angina-related symptoms that he had previously experienced.
Case 4
Screening family members for raised Lp(a) levels
Lp(a) levels are co-dominantly inherited and the LPA gene is located on chromosome 6 (6q26-27).8 Therefore, the offspring of affected individuals have a 50% chance of inheriting the LPA gene and consequently raised Lp(a) levels, raising the potential of family screening for this condition. However, there are some caveats associated with screening asymptomatic family members for raised Lp(a) levels, not least of which is the current absence of scientific evidence that reducing raised Lp(a) levels is beneficial in the primary prevention of ASCVD. Prospective CV outcome trials to confirm that lowering Lp(a) levels is beneficial in secondary prevention are ongoing and results are awaited. Furthermore, there are currently limited treatment options to selectively reduce Lp(a) levels (although clinical trials into future potential options are ongoing). The clinical relevance of raised Lp(a) levels remains uncertain and can cause anxiety amongst diagnosed individuals.
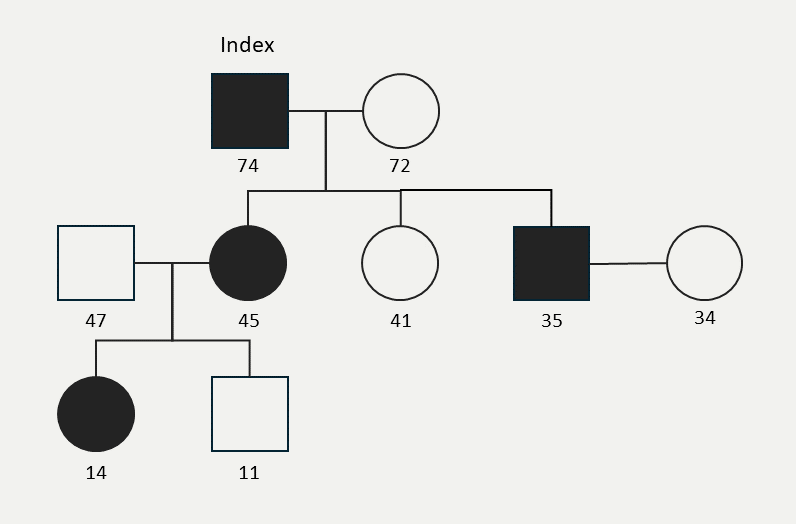
A gentleman with no other CV risk factors, aside from a strong family history of premature CVD and significantly raised Lp(a) levels of 1,050 mg/L at initial assessment (currently 240 nmol/L using the recommended isoform insensitive assay), had presented prematurely with ischaemic heart disease aged 52 with an acute coronary syndrome initially requiring balloon angioplasty to the circumflex artery. Subsequently he required multiple percutaneous coronary intervention (PCI) revascularisation procedures in different coronary territories until the age of 67, with the most recent being PCI to the left anterior descending artery. Stabilisation in the progression of CAD was achieved once he was treated with regular LA at the age of 67, after which no further revascularisations have been required to date.
As a father of three and grandfather of two to date, he was provided with comprehensive information regarding the genetic basis of raised Lp(a) levels and it was recommended that he inform his family members and encourage them to seek testing. Owing to the current lack of formal guidance on screening asymptomatic family members for raise Lp(a), no informational resources were available to share with his family members, representing an avenue for future improvement. When the patient was 74 years old, his eldest child, a daughter (45 years) underwent testing and was detected to have a raised Lp(a) level of 195 nmol/L. Amongst her two children, the eldest has been found to have a raised Lp(a) level of 180 nmol/L (14 years) but her younger child (11 years) is unaffected. The gentleman’s second child is unaffected and his third (a 35-year-old male) has been found to have a significantly raised Lp(a) level of 312 nmol/L and has not yet had children of his own. Neither of his two affected children have yet developed CVD and both remain asymptomatic. His affected daughter has no other CV risk factors and has an otherwise normal lipid profile. His son has a concurrently raised LDL-C level of 4.3 mmol/L, was previously a smoker and had a raised body mass index (BMI) of 31.8 kg/m2. In the absence of an evidence-based mandate to implement Lp(a)-lowering therapy for primary prevention, the clinical focus was to control his LDL-C levels, which was achieved with a combination of low-dose statin therapy, diet and lifestyle changes, including quitting smoking and reaching a healthy BMI of 24 kg/m2.
This family history highlights the genetic preponderance of raised Lp(a) levels and the unresolved management dilemmas amongst family members who are also detected to have raised Lp(a) levels, particularly in primary prevention. The genetic family pedigree is depicted in figure 4.
Case 5
Current challenges in managing raised Lp(a) levels
Limited availability of effective treatment options for lowering raised Lp(a) levels, in the absence of specific licensed therapies for this purpose, pose another major challenge in day-to-day management of affected individuals. Currently LA, a selective lipid-lowering extracorporeal treatment, remains the most effective available means of lowering Lp(a) levels and may be considered in patients with progressive CVD. However, due the invasive nature of this therapy and requirement to be performed frequently on a weekly or fortnightly basis, many patients remain reluctant to pursue this costly, time-consuming and invasive option.
A 55-year-old lady with a significantly raised Lp(a) level of 340 nmol/L (or 1,520 mg/L using the historic assay) presented with an acute coronary syndrome when she was 50 years old, requiring an urgent triple CABG surgery. Thereafter at the age of 51, she required recurrent revascularisation and underwent PCI to her RCA, followed by a second PCI due to in-stent restenosis in her RCA at the age of 54. Recent angiography shows that the left internal mammary artery graft is patent and both vein grafts are occluded. Currently, her lipid levels are being treated with rosuvastatin 20 mg as she is unable to tolerate higher doses, combined with a PCSK9 inhibitor in the form of evolocumab 140 mg subcutaneously every fortnight. Her Lp(a) level remains residually raised at 280 nmol/L.
In view of her progressive CVD, recurrent revascularisation procedures and raised Lp(a) levels, she would be eligible for LA therapy, for which she has been fully counselled regarding the advantages and disadvantages. After careful consideration, she has opted to decline LA therapy due to its invasive and frequent nature and the potential disruption this would cause to her occupation and quality of life. Instead, she has elected to participate in a randomised controlled trial evaluating potential future treatment options.
Conclusion
In summary, a raised Lp(a) level continues to gain momentum as an important, independent CV risk factor and therapeutic target. However, these real-world cases serve to illustrate that challenges remain with regards to screening Lp(a) levels in high-risk individuals and guidance on familial screening is lacking, for which further research is required. Treatment options currently remain limited; the lack of currently available licensed Lp(a)-lowering therapies must be communicated sensitively when giving a diagnosis of raised Lp(a) to minimise feelings of vulnerability in patients. LA represents a relatively effective Lp(a)-lowering option and can reduce CV symptoms and disease progression in selected individuals, as demonstrated in some of these cases. However, due to its invasive nature, high cost, limited accessibility and lack of NICE reimbursement at the current time, it is not a suitable therapy for all patients. Further research into specific Lp(a)-lowering therapies for both secondary and primary prevention of ASCVD is needed.
Key messages
- Lp(a) continues to gain recognition as an important independent cardiovascular (CV) risk factor and therapeutic target
- Global challenges exist with regards to screening Lp(a) levels in high-risk individuals and guidance on familial screening is limited due to gaps in the existing evidence base
- Treatment options remain limited, pending ongoing clinical trials to evaluate specific Lp(a)-lowering therapies
- Lipoprotein apheresis can reduce CV symptoms and disease progression in selected individuals; however, accessibility to this expensive treatment option remains limited and many patients are reluctant to pursue this therapy due to its invasive, time-consuming and frequent nature.
Conflicts of interest
TZK has received an honorarium for work on this supplement.
Tina Z Khan
Consultant Cardiologist
Department of Cardiology
Harefield Hospital, Royal Brompton & Harefield NHS Foundation Trust Hospital
Part of Guy’s and St Thomas’
Hill End Road, Harefield, UB9 6JH
([email protected])
Articles in this supplement
Introduction
Lipoprotein(a) in atherosclerotic heart disease and familial hypercholesterolaemia
Lipoprotein(a) measurement – how, why and in whom?
Clinical utility of lipoprotein(a): an interventionist’s perspective
References
1. Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: a review. JAMA Cardiol 2022;7:760–9. https://doi.org/10.1001/jamacardio.2022.0987
2. Catapano AL, Tokgözoğlu L, Banach M et al; Lipid Clinics Network Group. Evaluation of lipoprotein(a) in the prevention and management of atherosclerotic cardiovascular disease: a survey among the Lipid Clinics Network. Atherosclerosis 2023;370:5–11. https://doi.org/10.1016/j.atherosclerosis.2023.02.007 [Epub online ahead of print]
3. Alonso R, Andres E, Mata N et al. Lipoprotein(a) levels in familial hypercholesterolemia. J Am Coll Cardiol 2014;63:1982–9. https://doi.org/10.1016/j.jacc.2014.01.063 [Epub online ahead of print]
4. Vuorio A, Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J Intern Med 2020;287:2–18. https://doi.org/10.1111/joim.12981
5. Kraft HG, Lingenhel A, Raal FJ, Hohenegger M, Utermann G. Lipoprotein(a) in homozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2000;20:522–8. https://doi.org/10.1161/01.atv.20.2.522
6. Parish S, Tomson J, Wallendszus K et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12. https://doi.org/10.1056/NEJMoa1300955
7. Khan TZ, Hsu LY, Arai AE et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J 2017;38:1561–9. https://doi.org/10.1093/eurheartj/ehx178
8. Jacobson TA. Lipoprotein(a), cardiovascular disease and contemporary management. Mayo Clin Proc 2013;88:1294–311. https://doi.org/10.1016/j.mayocp.2013.09.003
