An 86-year-old woman presented with a six-month history of severe peripheral oedema and limiting breathlessness. A dual chamber pacemaker had been implanted 12 years earlier for complete heart block, and she had recently been prescribed amiodarone for paroxysmal atrial fibrillation. Previous echocardiography had demonstrated a small hypertrophied left ventricle with an end-diastolic diameter of 3.9 cm and good systolic function.
A clinical diagnosis of cardiac failure was made and she was admitted under the medical team for in-patient treatment. High-dose intravenous diuretics were administered, but despite prolonged treatment over a 10-day period, she remained severely oedematous, and due to her lack of progress, she was referred for cardiology review.
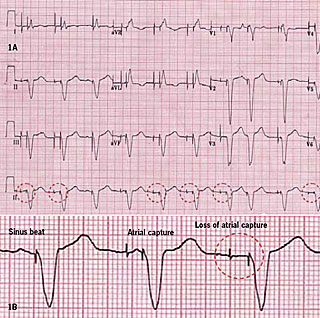
Her blood pressure was 95/60 mmHg and there were intermittent cannon waves on examination. Although her electrocardiogram showed atrioventricular sequential pacing with what appeared to be the occasional tracking of sinus beats, there were some early paced ventricular beats and other periods of ventricular safety pacing. Close inspection revealed no identifiable P waves following the atrial pacing artefact (figure 1 – dashed rings), and dissociated P waves at other points within the cardiac cycle. Atrial sensing was intact, though, and there was no evidence of retrograde (VA) conduction. These findings were consistent with loss of atrial capture resulting in atrioventricular dyssynchrony, suggesting that pacemaker syndrome had been responsible for her presentation.
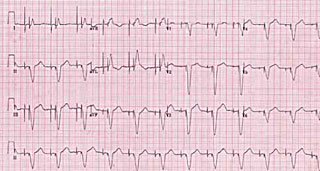
Pacemaker interrogation was undertaken and confirmed an increased atrial threshold of 2.9 V at 0.4 ms, above the current output of 2.5 V. The atrial lead impedence was normal at 730 Ohms, ventricular lead parameters and battery function were satisfactory, and there was near continuous atrial and ventricular pacing. Chest radiography showed satisfactory lead integrity and positioning, with the ventricular lead sited at the cardiac apex. The atrial output was increased resulting in consistent atrial capture (figure 2), and her oedema subsequently resolved completely over the following week, allowing for complete withdrawal of her intravenous diuretics.
Discussion
There is no single universal definition of pacemaker syndrome, but this generally refers to the spectrum of symptoms and signs, ranging from non-specific lassitude to severe hypotension and cardiac failure, which result from atrioventricular dyssynchrony, and the fall in cardiac output, elevation in filling pressures and reflex vasodilatation that accompany this. Classically, pacemaker syndrome occurs in conjunction with single chamber ventricular pacing, and may lead to severe and debilitating symptoms when retrograde (VA) conduction is present, as is frequently the case in patients with isolated sinus node disease.1
In the era of physiological dual chamber pacing, the diagnosis is often forgotten, but pacemaker syndrome may still occur when atrial latency (i.e. a prolonged delay between the atrial pacing artefact and paced P wave),2 or an inappropriately long paced atrioventricular delay in the presence of a resting tachycardia, are present. These situations result in atrial contraction occurring when the atrioventricular valves are closed, thus resulting in highly elevated venous pressures.
As in the current case, pacemaker syndrome can also occur with loss of atrial capture, and this in turn may be precipitated by anti-arrhythmic drugs, such as amiodarone, which increase the pacing threshold. In this setting, the patient essentially reverts to single chamber ventricular pacing with atrioventricular dyssynchrony. Withdrawing anti-arrhythmic drugs may be appropriate, and if sensing is intact, then reducing the lower rate limit for pacing may correct the problem.
In this particular case, it was felt more appropriate to increase the pacing output, as she had had previous symptomatic atrial fibrillation, and either sinus bradycardia or cessation of amiodarone would have made further episodes likely. Pacemaker longevity is a potential issue, however, and these measures, or even placement of a new atrial lead, might eventually need to be considered at the time of generator replacement, if this is required because of premature battery depletion.
This case was unusual because of the severity of the patient’s cardiac failure and its resistance to diuretic therapy. A detailed study for diastolic dysfunction was not undertaken, but in the setting of left ventricular hypertrophy it is likely that left ventricular compliance was significantly reduced and that she was dependent on the ‘atrial kick’ for adequate ventricular filling. We have seen a similar patient who developed severe cardiac failure due to loss of atrial capture following aortic valve replacement, in whom there was an acute rise in the pacing threshold post-operatively.
The diagnosis of pacemaker syndrome requires a high index of suspicion and the correlation of symptoms or relative hypotension with periods of ventricular pacing. The electrocardiogram should be inspected carefully for loss of atrial capture, particularly when anti-arrhythmic drugs which increase the pacing threshold have been prescribed. Appropriate programming and pacemaker prescription at the time of implantation are important, and single chamber ventricular pacemakers should be avoided especially in patients with isolated sinus node disease or left ventricular hypertrophy.3
Conflict of interest
None declared.
References
- Lamas GA, Ellenbogen KA. Evidence base for pacemaker mode selection: from physiology to randomized trials. Circulation 2004;109:443–51.
- Grant SC, Bennett DH. Atrial latency in a dual chambered pacing system causing inappropriate sequence of cardiac chamber activation. Pacing Clin Electrophysiol 1992;15:116–18.
- Castelnuovo E, Stein K, Pitt M, Garside R, Payne E. The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation. Health Technol Assess 2005;9:iii, xi–xiii, 1–246.
