What is a surrogate end point? Research, particularly intervention trials, can only be appropriately interpreted with pre-defined end points. The hypothesis being tested will be either proven or disproven by comparing the occurrence of the defined end points in the intervention and comparator groups. ‘Hard’ clinical end points are definable, identifiable and clinically important outcomes that directly reflect states of health, disease and illness.
A ‘surrogate marker’ can be used in place of a hard clinical end point. It is assumed to be representative of the ultimate clinical end point goal – an increased incidence of the surrogate marker will necessarily be followed by an increased frequency of the hard end point. In some cases, the surrogate marker is an intermediate step in the direction of the hard end point, being linked through pathophysiological processes, while in others it merely identifies a person at increased risk of the hard end point.
A surrogate marker is measurable, recordable and often changes more rapidly and more sensitively than the hard end point in response to interventions. To be useful, the surrogate marker must be affected by the study intervention. Usually several surrogate markers are pre-defined in clinical trials as primary and secondary end points. Because surrogate end points tend to occur earlier and more often than ‘hard’ end points, power calculations in interventional studies are often based upon expected numbers of surrogate end points, and these become the primary end points of the study.
Use of surrogate markers
Surrogate markers are important for the research, development and registration of new drugs, for example in evaluating drug dose-response and optimal doses, determining clinically relevant efficacy, toxicity and the safety/tolerability profile.
Surrogate markers can be used in different ways:
- as an aid to diagnosis
- as a tool for staging disease
- as an indicator of disease status
- as a measure to predict and/or monitor clinical response to an intervention.
- Surrogate markers are generally more cost-effective or more easily measured than hard clinical end points.
Clinical research and practice
Discussants considered a number of specific surrogate markers for cardiovascular disease and diabetes in the field of clinical research. Left ventricular hypertrophy (LVH), for example, as measured using ECG is an indicator of severity of hypertension. Regression of LVH may be used to estimate the efficacy of antihypertensive agents, while LVH itself is used as an indicator of risk for stroke.
Cholesterol reduction is used as a surrogate marker of one factor in reducing risk of coronary artery disease and cardiovascular mortality. Intravascular ultrasound (IVUS) was used to measure changes in atheroma burden as a surrogate marker for cardiovascular events in the REVERSing Atherosclerosis with aggressive Lipid-lowering therapy trial (REVERSAL).1
‘Surrogate markers represent intermediate steps to a hard end point or to identify individuals who are at risk’
Both blood pressure and urinary albumin excretion may be used as surrogate markers for cardiovascular disease in patients with hypertension and type 2 diabetes, as in the Candesartan And Lisinopril Microalbuminuria (CALM) trial,2 for instance. Changes in glycosylated haemoglobin (HbA1c) are used as a surrogate marker for microvascular complications in diabetes.

Numerous laboratory parameters are routinely used as surrogate markers in clinical practice (see table 1).
‘They are used extensively in clinical trials and clinical practice, and can be used to drive national audits and to monitor performance’
Performance management strategies
Surrogate measures are also important in practice as they can be used to drive national audits, and they are assessed in the Quality and Outcomes Framework (QOF).3 Key QOF targets for general practitioners involve measurement and recording of parameters to manage coronary heart disease, hypertension and diabetes, among many other conditions. Waiting times, average length of hospital stay and readmission rates are also often used as markers of quality of care.
Myocardial Ischaemia National Audit Project (MINAP) guidelines/targets, such as door-to-needle time and call-to-needle time, are used as measures of earlier treatments and surrogates for better outcomes. The proportion of patients discharged from hospital who are taking aspirin, statins and other treatments may be used as surrogate markers of lower recurrence of events.4
Validation of surrogate end points
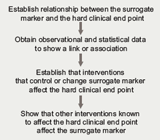
In order to assess/validate the usefulness of a surrogate marker in determining the effectiveness of a particular treatment, the Group recommended that various parameters should be determined (figure 1).
Ideally, the surrogate marker should be easier to measure, show more rapid results and be more cost-effective to measure than a hard end point. It should also have low inter-observer variability and should be reliable with repeated measures, especially in chronic diseases.
Other clinical surrogate markers
Some surrogate markers have ultimately been shown to be unrelated to the clinically meaningful end point or, despite an association, to have limited clinical value. The Group identified these as:
Ventricular ectopic activity, as measured by ventricular premature beats. This was used as a surrogate marker for sudden death in post-myocardial infarction patients in the Cardiac Arrhythmia Suppression Trial (CAST). The trial demonstrated a decrease in ventricular ectopic activity but mortality rates increased in both treatment groups.5
Microalbuminuria and proteinuria, although surrogate end points for target organ damage due to hypertension,6,7 were felt by the Group to be of limited practical value.
High-sensitivity C-reactive protein is a surrogate marker for inflammation but it is not generally used in clinical practice.8
References
- Schoenhagen P, Tuzcu E, Apperson-Hanse C. Determination of arterial wall remodelling during lipid-lowering therapy; serial intravascular ultrasound observations from the reversal of atherosclerosis with aggressive lipid-lowering therapy (REVERSAL) trial. Circulation 2006;113:2826–34.
- Mogensen CE, Neldam S, Tikkanen I et al for the CALM study group. Randomised controlled trial of dual blockade of the renin-angiotensin system in patients with hypertension, microalbuminuria and non-insulin dependent diabetes mellitus: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000;321:1440–4.
- Department of Health. Quality and Outcomes Framework.
- Myocardial Ischaemia National Audit Project (MINAP). Royal College of Physicians of London. http://www.rcplondon.ac.uk/college/ceeu/ceeu_ami_home.htm. Accessed 25th February 2008.
- Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324(12):781–8.
- Marre M. Microalbuminuria and prevention of renal insufficiency and cardiovascular diseases. Am J Hypertens 1998;11:884-6.
- Williams ME. Diabetic nephropathy: the proteinuria hypothesis. Am J Nephrol 2005;25:77–94.
- Danesh J, Wheeler JG, Hirschfield GM et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–97.
