The Transcatheter Cardiovascular Therapeutics 2009 meeting was held in San Francisco, USA, from September 22nd – 25th.
COGENT: no clinically relevant interaction between omeprazole and clopidogrel
The strongest evidence yet that there is no clinically relevant adverse reaction between the proton pump inhibitor, omeprazole, and clopidogrel has come from the results of the COGENT (Clopidogrel and the Optimisation of Gastrointestinal Events) trial – a randomised trial of omeprazole versus placebo in patients taking clopidogrel.
Results showed no increase in the risk of cardiovascular events with omeprazole, but the drug was associated with a benefit in terms of reduced gastrointestinal (GI) effects. This should put an end to speculation that omeprazole interferes with the action of clopidogrel, which has been suggested in platelet function tests and observational studies. Presenting the data at the meeting, Dr Deepak Bhatt (VA Boston Healthcare System, Boston, USA) said: “Platelet assays and observational data are not a substitute for randomised controlled trial data”.
The trial tested a combination of omeprazole 20 mg and clopidogrel 75 mg versus clopidogrel alone in patients requiring clopidogrel for at least 12 months, e.g. following an acute coronary syndrome event or stent implantation. The study was aiming to recruit about 5,000 patients but was stopped early after 3,627 patients had been enrolled because the sponsor, Cogentus Pharmaceuticals, declared bankruptcy.
After a follow-up of 390 days, results showed almost identical rates of cardiovascular (CV) events – CV death, non-fatal myocardial infarction, revascularisation, or ischaemic stroke – in both groups. There were 67 such events in the placebo group and 69 in the omeprazole group. But gastrointestinal (GI) events (upper-GI bleeding or GI pain with underlying erosive disease), were significantly higher in the placebo group.
CURRENT STEMI PCI: doubling dose of clopidogrel beneficial in STEMI
Further subgroup analysis from the CURRENT OASIS 7 trial has shown that doubling the loading and maintenance dose of clopidogrel is beneficial in ST-segment-elevation-myocardial infarction (STEMI) patients undergoing primary percutaenous coronary intervention (PCI).
The CURRENT OASIS trial, presented at the recent European Society of Cardiology meeting in Barcelona, Spain, showed that doubling the loading dose of clopidogrel and the maintenance dose for seven days was associated with a reduction in ischaemic events and stent thrombosis without increasing bleeding, in acute coronary syndrome patients presenting to an emergency department and scheduled to undergo an early invasive strategy.
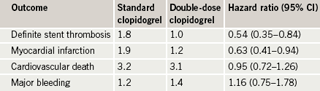
Of the original 17,232 patients undergoing PCI in the main study, 6,346 patients presented with STEMI, a significant majority of whom underwent primary PCI. This subgroup analysis focussed on these STEMI patients. It showed reductions in major cardiovascular events and stent thrombosis in the double dose clopidogrel group without an increase in bleeding (table 1).
In brief
Twelve-month results with bioengineered stent
Final 12-month clinical data from the e-HEALING clinical study showed that OrbusNeich’s Genous™ Bio-engineered R stent is safe and effective, including in the treatment of patients with diabetes.
The study evaluated 4,987 of the 4,996 enrolled patients and found a target vessel failure rate of 8.1% at 12 months with a follow-up rate of 92%, a target lesion revascularisation rate of 4.4%, a major adverse cardiac events rate of 7.7%, a subacute thrombosis rate of 0.5% and a late stent thrombosis of 0.3%. In a second study announced at the meeting, the stent was also shown to be safe in a prospective registry of 652 consecutive ST-elevation myocardial infarction patients.
Stent head-to-head studies
Abbott’s Xience V™everolimus-eluting stent was shown to be superior to Boston Scientific’s Taxus Express 2™ paclitaxel-eluting stent at one year in the SPIRIT IV study carried out on 3,690 patients including those with diabetes. The Xience™ stent showed 38% reduction in target lesion failure and a 46% reduction for target lesion revascularisation compared to the Taxus™ stent.
Stent thrombosis at one year was reduced by 80% with the Xience™ stent compared to the Taxus™ stent. The former demonstrated an observed 74% reduction in the Academic Research Consortium definition of definite/probable stent thrombosis at one year.
There was no difference in efficacy as measured by target vessel failure between Medtronic’s Endeavor™ zotarolimus-eluting stent and Boston Scientific’s Taxus™ paclitaxel-eluting stent at three years of patient follow-up in the ENDEAVOR IV clinical trial, which randomised 1,548 patients to either stent.
Long-term data, however, showed differences in measures of safety. Patients treated with an Endeavor™ stent had a statistically significant 48% (p=0.004) lower risk of myocardial infarction or cardiac death than patients treated with a Taxus™ stent, and a 91% (p=0.004) reduction in the risk of very late stent thrombosis.
