PCR London Valves 2014 was a dynamic meeting outlining multiple technological advances and novel concepts. Held in London from 28th September 2014 – 1st October 2014, the main discussion topics included transcatheter aortic valve implantation (TAVI) for intermediate risk and younger patients, refining known complications of the procedure, novel device technology and advances in transcatheter mitral valve implantation (TMVI). In a series of discussions and live case presentations, audience participation was actively encouraged. The ‘React@PCR’ system enabled audience members to post questions and comments for the panel of each session via the PCR London Valve App. Dr Katie O’Sullivan reports
TAVI for asymptomatic and intermediate risk patients
Indications for transcatheter aortic valve implantation (TAVI) are a critical aspect of determining the future of the therapy and this became a recurrent theme of the meeting. Current guidelines do not support the use of TAVI for those with asymptomatic aortic stenosis, as argued by Dr Bernard Prendergast (John Radcliffe Hospital, Oxford). There are some scenarios, however, where TAVI for asymptomatic aortic stenosis is appropriate, such as an immobile patient in whom symptoms have not appeared, or a frail patient with rapid progression of the condition.
To date, there have been three propensity matched European studies comparing TAVI to surgical aortic valve replacement in moderate risk patients: the OBSERVANT study, a study by Piazza et al. and a study Latib et al. Results from all studies have demonstrated equivocal mortality incidence for surgery and TAVI at 30 days; two randomised controlled trials – PARTNER 2 and SURTAVI – are ongoing. Until these are completed, TAVI is not indicated in those patients at intermediate surgical risk.
What’s new?
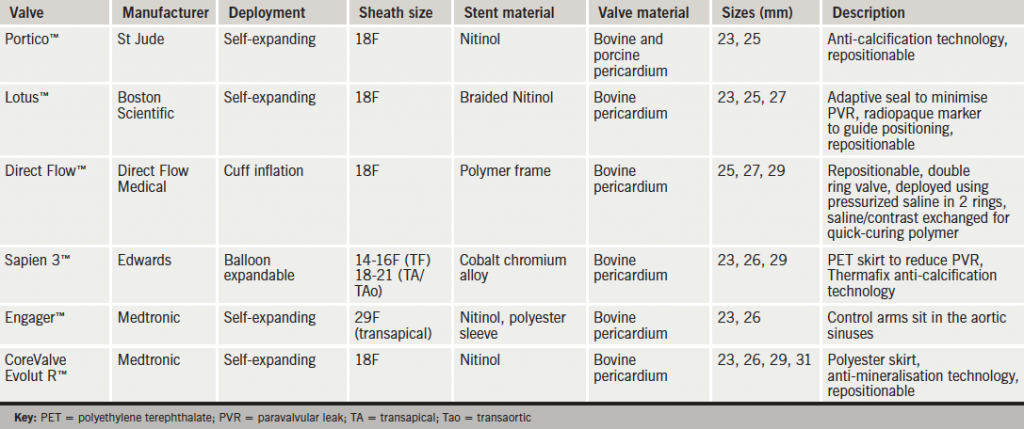
Dr Ganesh Manoharan (Royal Victoria Hospital, Belfast) gave an update on new TAVI devices (see table 1). Innovations include the St Jude Portico™ self-expanding nitinol valve with anti-calcification technology and a retrievable/repositionable design. Boston Scientific’s Lotus™ valve is a fully-retrievable valve comprised of a woven nitinol designed to minimise the incidence of paravalvular leak. Direct Flow Medical have designed a novel prosthesis with a fully retrievable bovine pericardial device which is first positioned and then held in place by exchanging saline within the valve rings for a quick-curing polymer which then solidifies in the aortic position. Updates of the two ‘workhorse’ TAVI valves in use to date are the Sapien 3™ balloon expandable valve from Edwards with a new skirt to minimise paravalvular leak, and the Medtronic Engager™ self-expanding prosthesis.
Refining TAVI
Paravalvular regurgitation
Paravavluar regurgitation following TAVI is an independent predictor of mortality and typically occurs because of valve under sizing, blocked apposition due to extensive valve calcification, or positioning of the valve too low or high. While rates have steadily declined since the advent of TAVI and newer device types have been designed to minimise its occurrence, it remains an issue. Dr Thierry Lefèvre (Institut Hospitalière Jacques Cartier, Massy, France) outlined strategies for prevention of valve under sizing. Specifically, this requires accurate valve selection and full inflation when using balloon-expandable TAVI devices. He highlighted the importance of optimum view and valve positioning at the time of deployment and the advantages of post-dilation in the minimisation of paravalvular regurgitation.
Stroke
Stroke continues to be a clinically relevant problem and recent data suggest that ‘silent’ cerebral infarcts are frequent and likely to impact on cognitive function. Dr Andreas Baumbach (Bristol Heart Institute, Bristol) described a number of embolic protection devices currently under investigation; the Embrella™ (Edwards), Triguard™ (Keystone Heart) and Claret Medical™ (Claret Medical) devices. Initial results are promising but the results of a number of clinical trials (Clean-TAVI, REFLECT, DEFLECT and PROTAVI-C) are awaited.
Vascular complications
Major vascular complications in early experiences with TAVI have occurred in approximately 11.9% of patients. Dr John Webb (St Paul’s Hospital, Vancouver, Canada) discussed how the introduction of new ‘ultra-low’ profile delivery systems is anticipated to reduce this incidence. Provisional results from the use of the Sapien 3™ device demonstrate a complication rate of just 4.2%.
How durable are TAVI valves?
Dr Peter Ludman (Queen Elizabeth Hospital, Birmingham) presented three- and five-year TAVI outcomes in high-risk patients with severe aortic stenosis from the UK TAVI registry. The registry allows “very accurate mortality tracking” in patients, he said. Commencing in 2007, it now has a total of 5,515 ‘all-comers’ (there were 1,534 procedures in 2013). Three-year survival was 61.2% and five-year survival (n=158) was 45.5%.
At five years, the demographic predictors of survival were:
- renal dysfunction
- respiratory dysfunction
- left ventricular dysfunction (left ventricular ejection fraction <30%)
- atrial fibrillation
- logistic EuroScore
- age
- coronary artery disease.
Stroke during or after TAVI was the only independent procedural predictor of survival. Device type, access route, paravalvular leak, pacemaker implantation, and vascular complications were not independent predictors of long-term mortality (at three or five years).
Professor Rudiger Lange (The German Heart Centre, Munich, Germany) presented the Munich Heart Hospital five-year results of 1,163 patients who had undergone TAVI. The group showed a 30−40% five-year survival rate with no alteration in effective orifice area or mean transvalvular gradient demonstrated up to six years. Some TAVI valve degeneration has occurred, albeit rarely, so longer-term durability of TAVI valves remains an unknown.
Personalised TAVI
As the number of valve prostheses and access routes expands, the issue of how to select the right access and valve prosthesis for the right patient has become a real clinical consideration. Professor Ian Meredith (MonaschHeart, Melbourne, Australia) analysed the specific issues with respect to valve sizing; balancing the risk of annular rupture with obtaining a good annular seal. He emphasised the importance of accurate sizing using computed tomography, three-dimensional echocardiography or magnetic resonance imaging data and also the critical importance of the Heart team analysing the data themselves rather than outsourcing it to a third party. In the same session, Dr Thomas Modine (Lille University Hospital, Lille, France) outlined specific access points for patients who had undergone previous coronary artery bypass surgery, suggesting carotid access offered a number of advantages.
The JenaValve™ (Jena Valve Technology) has a mechanism whereby native valve leaflets are captured in valve deployment and it is currently the only device licensed for use in aortic regurgitation. In addition, Mr John Hurley (Mater Private Hospital, Dublin, Ireland) described its specific selection for use in a patient with a prior mechanical mitral prosthesis due to its limited protrusion into the left ventricular outflow tract. The horizontal aorta can be challenging and Dr Thierry Lefèvre argued that self-centering devices, such as Sapien 3™, Lotus™ or Direct Flow Medical™, offer the best results in that setting. He also described that for patients with septal hypertrophy, self-expanding prostheses perform best (e.g. CoreValve™, Symetis Acurate™, Portico™).
An update on worldwide registry data
Dr Alec Vahanian (Bichat University Hospital, France) chaired the Great Valve Debate ‘Does the improvement of TAVI outcomes justify treatment of low-risk patients?’. Worldwide registry data were discussed from the PARTNER, GARY, and CoreValve™ Pivotal trials.
Mid-term follow-up data from the PARTNER trial have demonstrated a 21.8% mortality reduction with TAVI compared with standard therapy at five years. Valve hemodynamics remain excellent up to five years with no reported structural valve deterioration.
The German GARY registry now comprises 9,091 patients. Unadjusted mortality rates at one year are superior for transfemoral versus transapical TAVI (19.8 % vs. 24.9%, p<0.001). In the first large-scale registry comparing valve prostheses, incidence of paravalvular regurgitation (of >2) is 9.1% for CoreValve™ and 4.1% for the Sapien™ valve, with higher pacemaker insertion rates for CoreValve™ (28.9% vs. 12%).
An update on the US CoreValve™ Pivotal Trials was given by Dr Jeffrey Popma (Beth Israel Deaconess Medical Center, Boston, USA). He highlighted key findings including excellent valve haemodynamics at two years. Crucially, no neurological complications were found with valve post-dilation and the one-year mortality rate was 14.2%. Interestingly, there was a trend for reduced incidence of moderate paravalvular leak up to 12 months with a reduction from 15.3% to 11.9%.
Transcatheter mitral valve implantation
An exciting new area is that of transcatheter mitral valve implantation (TMVI). Main challenges lie in the complexity of mitral valve anatomy and the heterogeneity of disease. There are currently a number of TMVI prostheses under trial. During a keynote lecture, Dr Francesco Maizano (University Hospital, Zurich, Switzerland) discussed the complementary relationship between TMVI and mitral repair, emphasising patient selection as the key. Dr John Webb discussed results with the Tiara™ valve (Neovasc), which comprises a D-shaped self-expanding nitinol frame with bovine pericardial valve leaflets and an atrial skirt. Clinical experience is in the early stages and there have been three successful human implants to date with a feasibility study now underway.
Dr Lars Sondergaard (Copenhagen University, Denmark) presented a new self-positioning prosthesis, which anchors in the native mitral leaflets and has been tested and successfully deployed in four patients to date. It is anticipated a clinical trial will start in early 2015 with the enrollment of 100 patients across 10 sites. Mr Vinayak Bapat (Guy’s and St Thomas’ Hospital, London) reported outcomes of the Fortis™ valve (Edwards) in eight patients selected for compassionate reasons. The valve is self-expanding, circular and leaflets are made of bovine pericardial tissue. He demonstrated feasibility and excellent post-implantation haemodynamic results. A multi-centre prospective trial has commenced enrollment.
Clearly TMVI is at the very early stages of development and these early clinical results are very promising.
Katie O’ Sullivan
SpR Cardiothoracic Surgery
The Heart Team, Mater Private Hospital, Dublin, Ireland
