‘Yesterday’s problems, today’s solutions’ was the theme of the 2014 autumn meeting of the British Society for Heart Failure (BSH). A wide-ranging programme covered many topics from uncertainties, myths and dogmas to frightening calls and consultations including the heart failure patient with cancer. Drs Lindsey Tilling and Eleanor Wicks report some of its highlights.
Anaemia was one of several problems chosen for a case-based discussion in a session on common non-cardiac co-morbidities. Dr Callum Chapman (West Middlesex University Hospital) presented the case of an elderly patient with known coeliac disease who had undergone transcatheter aortic valve implantation, which resulted in a paraprosthetic leak and impingement of the mitral valve. Unfortunately despite medical management of the leak she presented to the elderly care service in New York Heart Association (NYHA) Class III heart failure and was extremely oedematous. Blood tests revealed an iron deficiency anaemia and a reduced transferrin saturation. The use of intravenous iron therapy improved symptoms greatly. Ultimately coeliac disease was found to be responsible for her anaemia. During the subsequent audience discussion, the point was made that intravenous iron is superior to oral administration in many ways, and is probably under-utilised.
Uncertainties, myths and dogmas
This session began by Dr Nigel Rowell (Cleveland Health Centre, Middlesbrough) confronting various demographic dogmas. He disputed that the prevalence of heart failure is on the decline, pointing out that data collection is subject to financial issues, errors in coding, and geographical differences. He disproved the suggestion that heart failure admissions are increasing but agreed that there is a high readmission rate, with the first seven to 10 days post-discharge being critical in determining readmission. He also did not agree that heart failure patients’ outlook is poor. He presented data confirming the incidence of heart failure is higher in men than women. While endorsing ischaemic heart disease as the number one cause of heart failure, he refuted the claim that heart failure with a preserved ejection fraction (HFPEF) does not exist.
Dr Paul Kalra (Portsmouth Hospitals NHS Trust) then shed light on the uncertainty surrounding sodium and water restriction in heart failure management. Although perceived as common sense, he questioned whether fluid restriction is good clinical practice. He presented a patient with severe left ventricular systolic dysfunction who became oedematous and hyponatraemic and suggested that guidance to restrict oral intake is ‘reasonable’ in fluid overloaded patients but that there is little strong evidence to support this rationale. Only small studies of patients who are not medically optimised have been undertaken, with variable sodium levels advised, and none have been performed in HFPEF.
A study by Aliti et al. of patients in acutely decompensated heart failure (ADHF) was discussed in detail. These patients found it very difficult to keep to a tight fluid restriction, had no significant weight loss after three days, and complained of thirst. In rats, sodium restriction has been found to increase aldosterone and promote fibrosis, indicating potential deleterious mechanisms, which result from such management in humans. The practical suggestions were to consider fluid restriction in ADHF patients with hyponatraemia in whom diuresis is difficult to control. Additionally patients should be advised to avoid excessive (>3 g/day) salt in their diet.
Anticoagulation in heart failure
The importance of anticoagulation in atrial fibrillation (AF) has been well recognised for many years. The presence of an impaired ventricle in a patient with AF makes an even stronger case for consideration of anticoagulation. The role of anticoagulation in patients with heart failure and sinus rhythm is much less well defined. This ‘uncertainty’ was discussed by Dr Lindsey Tilling (Royal Brompton and Harefield NHS Trust, London).
The recently published, large, randomised-controlled trial WARCEF (Warfarin and Aspirin in Heart Failure with Sinus Rhythm) excluded patients with a pre-existing anticoagulation indication. No difference was seen in the composite end point of intracerebral haemorrhage, ischaemic stroke or death from any cause, but the incidence of ischaemic stroke was significantly lower in the group receiving warfarin versus the group receiving aspirin. The absolute risk reduction, however, was small and this benefit was offset by the increase in bleeding events. European guidance is thus circumspect, stating that in the absence of an overall benefit of warfarin on rates of death and stroke with an increase in major bleeding, there is currently no compelling reason to routinely use warfarin for all heart failure patients in sinus rhythm. No real data exist on the use of novel oral anticoagulant agents (NOACs) in heart failure and sinus rhythm but some trial data of NOACs versus warfarin in AF suggest there may be less bleeding complications with NOACs. This may indicate a possible beneficial role if used in patients with heart failure without AF.
AF remains the undisputed strongest indication for anticoagulation but can we be confident that we have identified all of our patients with AF? A recent study found 40% of heart failure patients with implanted cardiac rhythm devices had atrial arrhythmias over the course of a year. Device therapy is likely to become increasingly prevalent amongst the heart failure population, particularly in the wake of the recently updated NICE (National Institute for Health and Care Excellence) guidelines. This should provide a good opportunity to screen patients for AF.
Finally, there remain several unanswered questions with little or no clinical evidence to support decision-making. These include the role of anticoagulation in HFPEF, and anticoagulation in heart failure of different aetiologies.
Lower cholesterol and HbA1C are better in HF
This myth was controversially exploded by Professor Andrew Clark (BSH President and Hull York Medical School). There is evidence that statins reduce morbidity and mortality in patients with coronary artery disease or with risk factors for coronary disease. But whether statins are of any benefit in heart failure, or indeed, may actually be detrimental, has been long debated. The CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) and the GISSI-HF [Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Heart Failure] trials were provided as evidence of lack of benefit.
CORONA investigated patients over 60 years of age with moderate to severe systolic heart failure of ischaemic origin who were randomised to rosuvastatin 10 mg once a day or placebo. Statin therapy had no effect on the composite end point of cardiovascular death, non-fatal myocardial infarct, or stroke. GISSI-HF enrolled patients over 18 years of age with heart failure of ischaemic and non-ischaemic aetiology. It too failed to demonstrate in the treated arm a reduction in time to death, and time to death or admission to hospital for cardiovascular reasons. During the discussion the speaker asked who would routinely stop statin therapy in their heart failure patients; approximately half of the audience indicated this was their practice.
The widely-held belief that higher HbA1C confers a worse prognosis was then addressed. Professor Clark went back to the original trials: DCCT (the Diabetes Control and Complications Trial), which was conducted in patients with type 1 diabetes, and UKPDS (United Kingdom Prospective Diabetes Study), a landmark study in type 2 diabetes. In the latter trial, the patient group receiving intensive glucose control had a significant reduction in seven diabetes-related events but there was no effect on mortality. Tighter control mainly resulted in a reduction of microvascular complications and there was no statistically significant reduction in macrovascular disease, including cardiovascular events. These results were similar to those of the earlier DCCT trial. The point was made that the Quality Outcomes Framework (QoF) rewards lowered glycosylated haemoglobin measurements and it was questioned whether this is appropriate, at least in terms of cardiovascular outcomes.
Finally, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study was discussed, which randomised patients with type 2 diabetes to a standard (target HbA1C of 7.0–7.9%) or intensified regime (target HbA1C <6.0%). Of these patients, 35% had a history of a previous cardiovascular event. The study was terminated prematurely after the intensified regime was found to confer a relative increase in mortality of 22% after a mean of 3.5 years. This study indicates that very tight control of HbA1C does not benefit any patient group, and should be borne in mind when managing our diabetes patients with heart failure.
Frightening calls and consultations
The patient with cancer
The audience was given a wake-up call by a presentation on the patient with cancer from Professor Theresa McDonagh (King’s College Hospital, London). She equated oncological therapeutic agents to “weapons of mass destruction”. Although anthracyclines and trastuzumab (Herceptin℗) have improved cancer survival rates dramatically over the last 20 years, she emphasised that the use of complex chemotherapy regimens have the capability to cause catastrophic myocardial ”collateral damage” in up to 25% of patients. Recent years have seen rocketing referral rates for cardiological assessments for those with either primary or metastatic malignancy, those undergoing active chemotherapy or radiotherapy or for survivors of cancer. Cardiologists are, therefore, not infrequently presented with a multitude of ‘car crash’ clinical scenarios within this patient cohort and faced with the stark cardiotoxic complications of oncological treatments, whilst being amidst a swarm of uncertainties. Many questions remain unanswered: Can you ‘cure’ the heart failure within 48 hours to enable the patient to proceed with their next dose of chemotherapy? Should chemotherapy agents be continued or stopped? Should agents be changed or reduced in dose?
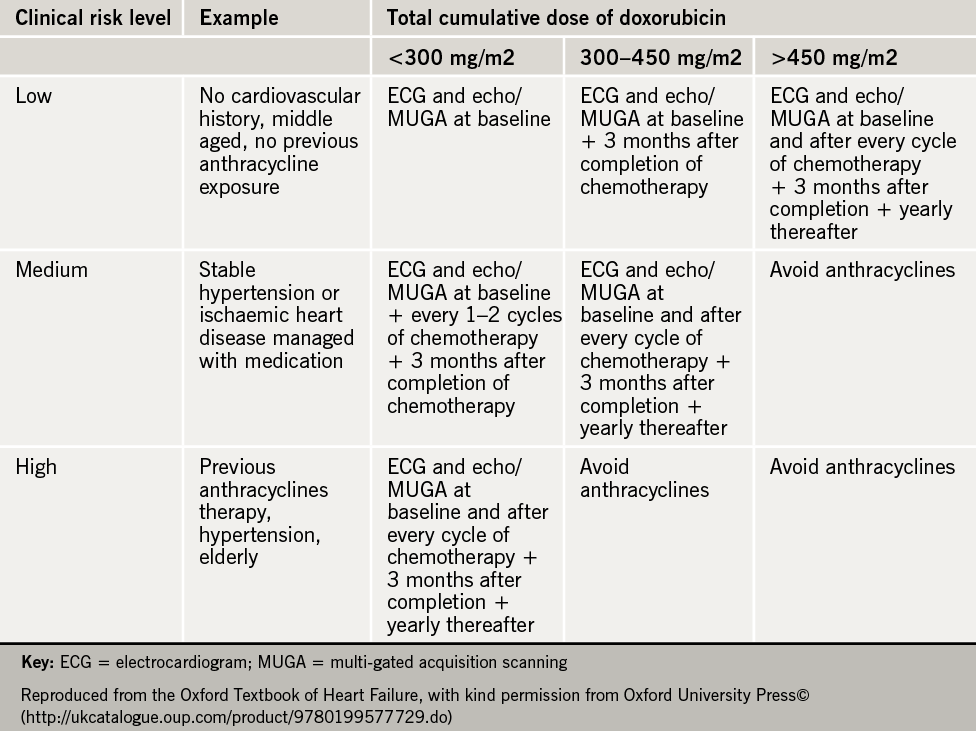
Professor McDonagh guided the audience through a systematic strategy for managing cardio-oncological patients. She emphasised the need for an enhanced awareness of the predisposing risk factors for cardiotoxicity (see table 1).

She described how identifying such risk factors enables the risk stratification of patients and offers the potential for disease prevention, early identification of disease and the delivery of guided, patient-specific management, according to risk (see table 2). Key recommendations for damage limitation and prevention of left ventricular dysfunction and heart failure when managing such cardio-oncological patients were described:
- The use of anthracyclines should be avoided in those with a left ventricular ejection fraction of less than 50% (LVEF <50%) or in those with a drop in LVEF >15% following treatment.
- Heart failure should be diagnosed and treated as for any non-cancer patient.
- Regular assessment and monitoring of disease should be performed consistently through the use of an electrocardiogram, an echocardiogram and the use of baseline and interval measurements of biomarkers throughout the treatment pathway (including brain natriuretic peptide and troponin). Alternative techniques to assess left ventricular function (e.g. cardiac magnetic resonance imaging or multi-gated acquisition scanning) can be adopted if used consistently.
Finally, despite a limited evidence-base, prevention of cardiotoxicity was described. Careful pre-treatment assessment and dosing to ensure utilisation of the minimal dose necessary for treatment were suggested. Targeted, focussed radiotherapy with shielding (known to improve protection of myocardial tissue and to prevent coronary disease) and delivering chemotherapy via an infusion rather than a bolus (and using liposomal derivatives with reduced toxicity), were noted to be important. Furthermore, prophylactic treatments using beta blockers, statins and aldosterone antagonists were all postulated to prevent disease development. Despite these management suggestions, Professor McDonagh emphasised that translational and clinical research is required to advance our understanding and evidence base for the use of such strategies in everyday clinical practice.
Dr Simon Williams (University Hospital of South Manchester) moved on to recap succinctly on the potential cardiovascular effects of chemotherapy and described the recognised clinical complications of anticancer therapies, including:
- Heart failure with ventricular dysfunction
- Fluid overload related to heart failure with preserved ejection fraction (particularly related to alkylating agents)
- Hypertension (related to anti-vascular endothelial growth factor agents);
- Ischaemia (particularly with 5-Fluorouracil [5FU])
- Arrhythmias (relating to QTc prolongation associated with tyrosine kinase inhibitors).
He reiterated that cardiotoxicity can occur as early as within three months of oncological therapy in up to 2% of patients or as late as up to 20 years following treatment in 5% of patients. He echoed that “prevention is better than cure,” emphasising the need for a detailed risk assessment in all patients prior to receiving oncological treatment to ensure the prescription of an individualised treatment strategy (with dose-adjustments, if necessary). He directed cardiologists to the European Society of Medical Oncology (ESMO) guidance for monitoring disease development and progression following the use of anthracyclines or trastuzumab.
He suggested that all patients undergoing oncological treatment should have an echocardiogram and troponin at baseline and at each treatment cycle thereafter (see figures 1 and 2 for anthracyclines or trastuzumab, respectively). Following the detection of abnormalities, he suggested that prognostic heart failure medications should be commenced and that closer monitoring at defined time frames should be instituted. Whilst the evidence base remains small, it was recommended that cardio-oncology patients with left ventricular systolic dysfunction (LVSD) be treated as per conventional national and international heart failure guidelines with lifelong prognostic heart failure medication. Closing remarks emphasised that collaborative, multidisciplinary efforts between cardiology and oncology are key to the management of these patients to ensure improved morbidity and mortality.
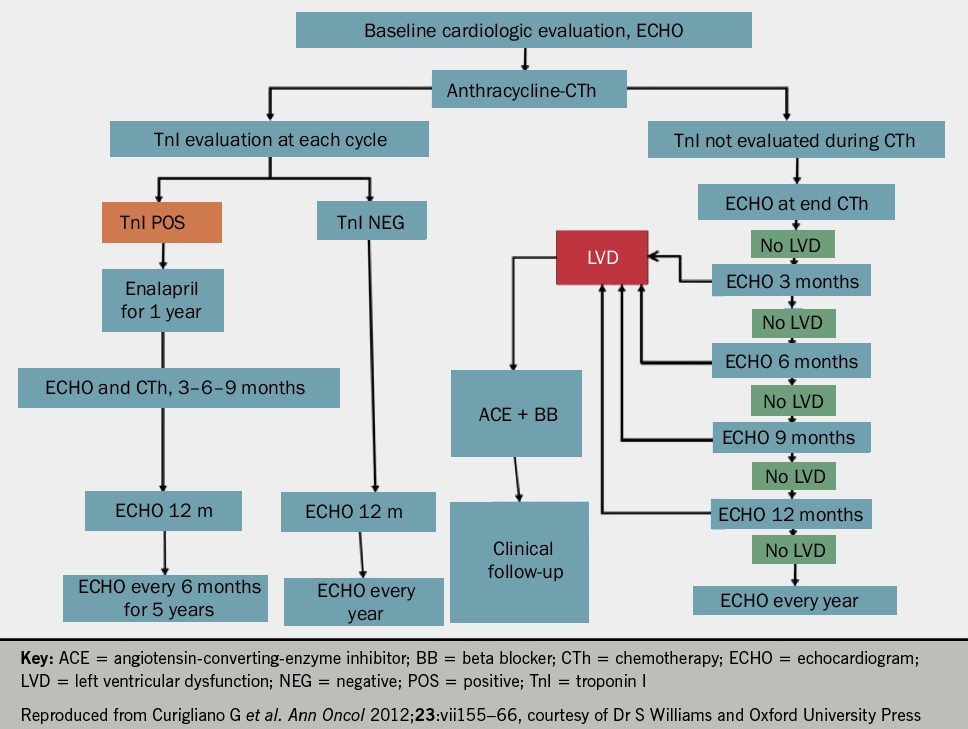
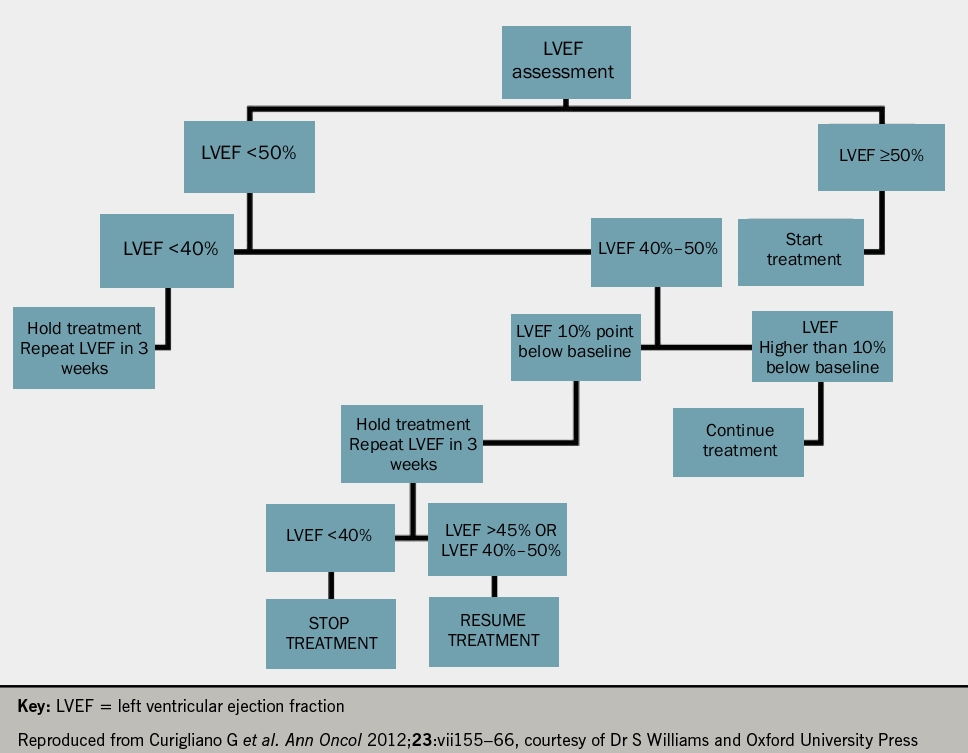
Devices and surgery
In a session examining devices and surgery “today and tomorrow,” Dr Ninian Lang (University of Glasgow) emphasised the importance of careful patient selection and the balance between risk versus benefit when considering and selecting device therapy. The EchoCRT (Echocardiography Guided Cardiac Resynchronisation Therapy) study demonstrated that in those with NYHA class III or IV symptoms, with an LVEF <30% and echocardiographic evidence of dyssynchrony, but a QRS duration of less than 130 ms, device implantation causes more harm than good. Additionally, MADIT-CRT (Multicentre Automatic Defibrillator Implantation Trial with Cardiac Resynchronisation Therapy) emphasised that in those with less severe symptoms (NYHA class I or II) with an LVEF of <30% and QRS duration >130 ms, only those in left bundle branch block (LBBB) demonstrated benefit from cardiac resynchronisation therapy-combined with a defibrillator (CRT-D) versus implantable defibrillator (ICD) alone. This suggests that QRS duration remains key in device selection irrespective of bundle branch morphology. Furthermore, it was demonstrated in MADIT-RIT (MADIT Randomised Trial to Reduce Inappropriate Therapy) that ICD shocks are disadvantageous, prompting a more conservative approach to device programming.
Dr Lang moved on to highlight the advantages of multipolar leads for improved pacing. These included the potential for improved programming flexibility and targetability for multi-point pacing, alongside their ability for variable stimulation to avoid scar tissue and reduced diaphragmatic twitch. He emphasised the issues surrounding endomyocardial lead placement including thromboembolic complications, as described in the ALSYNC (Alternate Site Cardiac Resynchronisation) study. In contrast to endocardial devices, he described the possibility of reduced device-related risks (both infection and implantation risks) with subcutaneous ICDs, as seen within the EFFORTLESS (Evaluation of Factors Impacting Clinical Outcome and Cost Effectiveness of the S-ICD) registry where 97% of a population of 456 patients with extracardiac devices remained free from complications at 360 days. He reiterated careful patient selection given the inability of such devices to provide bradycardia pacing or anti-tachycardia pacing (ATP).
Finally, Dr Lang emphasised the importance of getting the basics in device selection correct by optimally using our evidence base. Looking to the future, he suggested that we should focus our efforts to “make sense” of the vast quantities of data readily becoming available from these devices to enable a greater understanding for treatment and monitoring within this patient cohort.
In the pipeline
Dr Stephen Pettit (Papworth Hospital, Cambridge) eloquently took us to another level of complexity within the heart failure arena by emphasising the strategies for mechanical circulatory support (MCS) given the limitations in transplantation due to the shortage of donor hearts. Whilst the large, early ventricular assist devices (VADs) have been available since the 1960s, the newer and smaller implantable pumps with their extra-cutaneous drive-lines (such as the HeartWare™ HVAD [within the pericardial space] and HeartMate II™ devices [below the diaphragm]), offer advanced left ventricular (LV) support. Such devices are not without risk, and complications (as described in the INTERMACS study) including driveline infections, peripheral embolism (including intracerebral thromboembolic events and subsequent haemorrhage), device malfunction and device infection are common. Furthermore, devices are burdensome to patients, carers and health care providers alike and require effort for cleaning and dressing care and for management of the system, controller and batteries.
Nevertheless, Dr Pettit offered considerable hope with the potential for new generations of smaller devices (e.g. HeartMate III™ and MVAD HeartWare™ pumps), which offer the potential for easier device implantation without the need for sternotomy and potentially cardiopulmonary bypass. He described tiny circular pumps, including HeartWare X™, which whilst not achieving adequate flows for a left-sided system, offers the potential for right ventricular (RV) support. Similarly, the percutaneous short term VAD, the Impella CP™ device, has the capacity to provide further LV and RV circulatory support. He described future strategies to eliminate driveline infections through the use of an external jacket with coils and magnetic resonators to transfer power to the device. He emphasised the hope for newer devices to enable resumption of normal activities of daily living (such as washing and dressing) whilst offering improved recharging capabilities, which have previously restricted movement.
Other future advances within this arena include the use of materials, such as titanium, to reduce the thrombotic risk, and using stem cells to encourage a neo-intimal layer to grow on the device. In future there will be smaller devices for mechanical circulatory support that are effective, safe and affordable. For the majority of patients, the key is optimal medical management for heart failure. For a few patients, long-term device therapy (so-called ‘destination therapy’) may be appropriate, but there will have to be further consultation to allow the use of devices as destination therapy rather than just as a bridge to transplantation.
Dr Nawwar Al-Attar (Golden University Jubilee Hospital, Clydebank) then walked us through history from Gibbon’s heart and lung machine in 1953, to the first bridge to transplant in 1969, the first LVAD in 1977 and the total artificial heart (TAH) in 1981. He consolidated our understanding of first-, second- and third-generation mechanical circulatory support and described extracorporeal devices, reiterating the future – to be comprised of the smaller LVAD devices HeartMate III™, HeartMate X™, HeartWare MVAD™ and their potential for improved biocompatibility and durability. Dr Al-Attar described how the REACH trials’ extravascular aortic balloon pump can be used to provide aortic counterpulsation. Furthermore, given that 10−20% of patients have concomitant RV failure, he described how the BiVACOR® rotatory biventricular assist device and total artificial heart can be used to overcome the clinical challenges within this specific patient cohort. Finally, he described the advancements and use of the cold storage machine to supply the donor heart with blood and enable tests to be carried out to ensure the viability of donor hearts.
Professor Christian Latrémouille (Georges Pompidou Hospital, Paris, France) brought the meeting to its conclusion by describing the innovative surgical technological advancement on the horizon – ”the novel total integrated bioprosthetic heart”. This model has been proposed as a destination therapy with haemocompatibility. Whilst validated in animal models, Professor Latrémouille described how this therapy has been tried and tested in four patients to date. Clinical outcomes, so far, appear optimistic. Further research needs to validate the in vivo use of this potentially groundbreaking technique to establish whether it can provide true hope to our advanced heart failure communities.
Dr Lindsey Tilling,
SpR Cardiology
Dr Eleanor Wicks
SpR Cardiology
Royal Brompton & Harefield NHS Trust, London
Acknowledgement
The BSH gratefully acknowledges the support provided by the Friends of BSH: Bayer, Medtronic, Novartis, Pfizer, ResMed and Servier Laboratories.
BSH contact details
For further information about the BSH, please contact the society at: E: [email protected], www.bsh.org.uk or twitter @BSHeartFailure
Future BSH meetings
7th BSH Heart Failure Day for Revalidation and Training, 5 March 2015, Charterhouse Square, London
5th BSH Heart Failure Nurse Study Day, 6 March 2015, Charterhouse Square, London
18th BSH Annual Autumn Meeting – 26–27 November 2015, Queen Elizabeth II Conference Centre, London
Conflict of interest
None declared.

