Current transcatheter mitral valve techniques are at the beginning of an era of innovation before their full potential is realised. The broadening of available options for mitral regurgitation (MR) reduction is welcome and transcatheter mitral valve interventions provide complementary strategies in the drive for more safe and effective therapies for patients. In this article, the evidence and indications for MitraClip® are reviewed.
Introduction

Mitral regurgitation (MR) is increasingly prevalent in developed countries and represents a significant cause of morbidity and mortality. It affects 24% of adults with valvular heart disease and is present in 7% of the population over the age of 75 years.1,2 Significant MR is a complex condition and, left untreated, it leads to slow progressive deterioration. Up to 50% of patients with criteria for surgical intervention are not referred for surgery, largely due to advanced age, significant comorbidities and the presence of left ventricular (LV) dysfunction.3,4 This unmet clinical need has fuelled innovation in transcatheter approaches for the mitral valve.
Mitral valve anatomy and function
The mitral valve structure is complex. The dynamic, saddle-shaped annulus provides fibrous continuity between the anterior mitral valve leaflet and aortic valve cusps. The leaflets are supported by a subvalvular apparatus (including the chordae tendinae and papillary muscles) and are in proximity to the aortic valve, circumflex coronary artery, coronary sinus and the conduction system. The central position of the mitral valve, with valvular and subvalvular connections in the left ventricle, helps maintain left ventricular shape and chamber contractility.5 Mitral valve therapy requires attention to the ‘functional anatomy’ of the mitral valve complex,6,7 in order to avoid injury and complications.
Classification of MR and mitral valve pathology
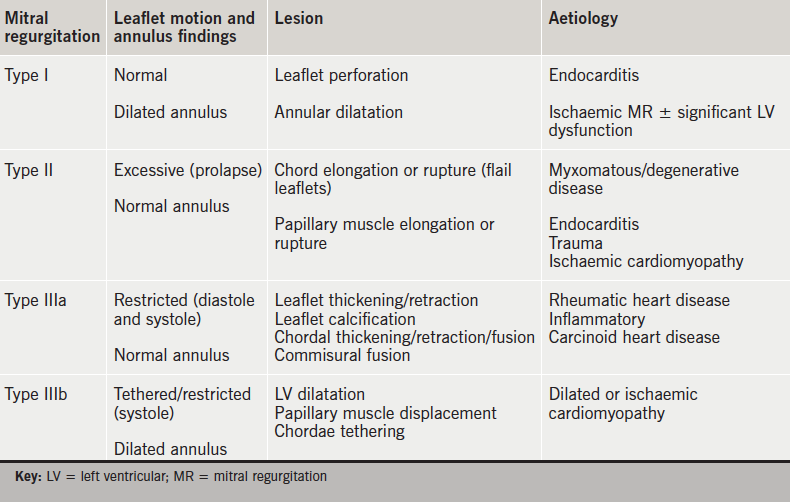
 MR is broadly classified into two main categories: primary valvular disease or organic MR (intrinsic valve lesions) and secondary or functional MR (a structurally normal mitral valve but ventricular remodelling causes downward papillary muscle displacement, leaflet tethering and annular dilatation). The Carpentier classification8 describes MR based on leaflet movement and provides a unifying language to describe mitral valve pathology based on echocardiographic findings (table 1). The posterior leaflet is divided into three segments or scallops: lateral P1, middle P2 and medial P3. The opposing A1, A2 and A3 segments of the larger anterior leaflet correspond to the posterior leaflet segments.
MR is broadly classified into two main categories: primary valvular disease or organic MR (intrinsic valve lesions) and secondary or functional MR (a structurally normal mitral valve but ventricular remodelling causes downward papillary muscle displacement, leaflet tethering and annular dilatation). The Carpentier classification8 describes MR based on leaflet movement and provides a unifying language to describe mitral valve pathology based on echocardiographic findings (table 1). The posterior leaflet is divided into three segments or scallops: lateral P1, middle P2 and medial P3. The opposing A1, A2 and A3 segments of the larger anterior leaflet correspond to the posterior leaflet segments.
Indications for mitral valve surgery
Guidelines recommend surgical repair or replacement for symptomatic patients (class I) with severe MR due to a primary valvular abnormality or asymptomatic patients with severe MR and LV dysfunction or enlargement.9,10 Surgery may also be considered an option for symptomatic patients (class IIb) with secondary (functional) MR.9,10 Mitral valve repair, when feasible, is almost universally regarded as the preferred method of MR correction (in non-rheumatic valves) over mitral valve replacement due to the advantages of even partial preservation of subvalvular chordae.11-14 Surgical repair techniques are defined by the patho-anatomy and physiology of MR,15,16 and provide a tailored approach to restoring normal mitral valve function.
Surgical repair results for primary or degenerative MR are excellent in experienced centres with procedural volume.17 Secondary or functional MR (FMR) remains a surgical challenge. Data have failed to clearly demonstrate survival benefit in these patients,18 and reported recurrence rates range from 15% to 60%.19,20 The pathophysiology of FMR is complex and the more contentious role of surgery reflects the underlying ventricular nature of the problem in a high-risk population. Only a third of patients with FMR are referred for surgery, and FMR is present in 90% of patients who are denied surgery.21,22
Challenges of transcatheter mitral valve techniques
The first case of percutaneous mitral valve repair was performed in 2003,23 and, yet, the clinical application of this approach has been more challenging compared with aortic valve stenosis.24 The greater complexity of mitral regurgitation, and the patient heterogeneity,25 presents greater challenges for the transcatheter approach, device development and, indeed, trial design.
MitraClip®
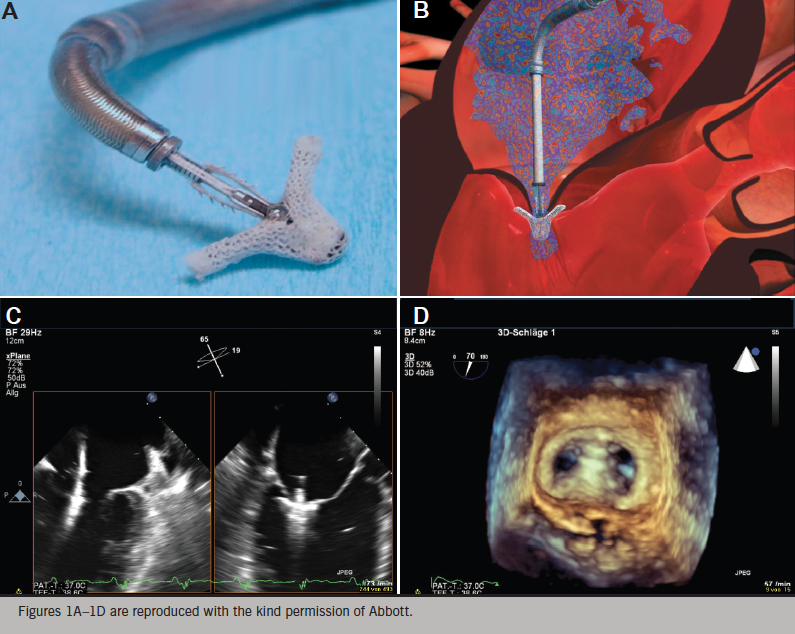
The MitraClip® (Abbott Vascular, Santa Clara, California, US) percutaneous repair system is the only transcatheter mitral valve therapy to undergo a pivotal randomised-controlled trial. It is based on the surgical procedure pioneered by Ottavio Alfieri in the early 1990s.26 Over 30,000 patients have been treated worldwide. This technique creates a competent double orifice valve by suturing the free edges of the middle portion of the anterior leaflet (A2) to the middle portion of the posterior leaflet (P2). To improve the durability of results, the Alfieri repair is typically combined with implantation of a partial band or complete annuloplasty ring, except in cases with a severely calcified mitral annulus.27
The MitraClip® is made of a cobalt–chromium alloy and covered with polypropylene fabric to promote tissue in-growth.28,29 It is a single-size clip device that has been used to treat patients with functional, mixed and degenerative MR. The Clip has a dual-arm structure, with grippers above the arms to assist with capture of the mitral valve leaflets and their approximation while the heart is beating.
The procedure
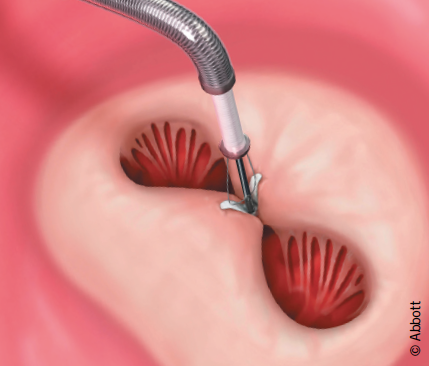
The procedure is approached via the femoral vein, and trans-septal access permits delivery of the catheter-based MitraClip® device into the left atrium (figure 1). All manoeuvres are performed under transoesophageal echocardiographic (TOE) visualisation. The device is positioned directly above the regurgitant jet and advanced across the mitral valve into the left ventricle. The Clip is retracted toward the mitral valve leaflets to engage the appropriate segments of the mitral valve. The grippers are dropped and arms of the Clip are closed. If the leaflet insertion visualised on TOE is acceptable, the degree of residual MR is assessed with the Clip fully closed. If reduction is inadequate, the Clip may be released and repositioned or a second clip may be implanted. The addition of Clips is guided by, among other factors, transmitral valve diastolic gradient, as a surrogate measure for potential development of mitral stenosis. After the Clip(s) are deployed, and the delivery catheter is removed from the patient, manual compression, use of a temporary subcutaneous suture, or placement of a percutaneous suture may be used to close the femoral vein access site.
The unique features of this procedure are:
- the clip is repositionable (if a decision is made not to deploy the Clip, it may be removed)
- real-time echocardiographic assessment of MR reduction is obtained
- it produces vertical coaptation of leaflets and is not simply an edge-to-edge repair
- surgical options are preserved.
Evidence base for MitraClip®
EVEREST II
MitraClip® is the first percutaneous device to be compared with surgery in a randomised-controlled trial (RCT). The pivotal EVEREST II (Endovascular Valve Edge-to-Edge REpair Study) phase II RCT comprising 279 patients was completed in 2008.30 It included patients with both degenerative and functional MR, although predominantly degenerative MR (73%). Surgery was superior for the primary outcome of freedom from death, surgery for mitral valve dysfunction, or ≥3+ MR. MitraClip® was safe with only 15% of patients experiencing a major adverse event compared with 48% of surgical patients (largely composed of transfusion ≥2 units). The degree of MR reduction was greater in the surgical patients, however, important clinical indicators, including heart failure functional status (New York Heart Association, NYHA), ejection fraction, LV dimensions and quality of life (QoL), improved in both groups. Nearly 80% of patients were free from 3+ or 4+ MR after Clip placement and did not require surgery in 12-month follow-up. The majority of MitraClip®-treated patients who required an additional procedure did so within the first six months after initial treatment.30 Clinically significant cases of mitral stenosis in the MitraClip® group was not observed.
Five-year follow-up of patients in the EVEREST II trial reported no increase in late MR recurrence compared with surgery.31 A good immediate result with MitraClip® showed excellent durability of the repair (at five years). The improved NYHA class was sustained and mortality rates were not different between the treatment arms at years one or five.
The rates of re-operation, or additional MitraClip® procedures, were no different between the two treatment groups after the first year. There did not appear to be a significant change in MR grade, ventricular function or dimension in follow-up, however, longer-term follow-up of the subset of patients with MR grade 2+ is ongoing.
Registry data
In Europe, CE mark was obtained in March 2008. ACCESS-EU (ACCESS-Europe A Two-Phase Observational Study of the MitraClip System in Europe) is a post-marketing registry of MitraClip® patients comprising 567 patients.32 These patients were more elderly and higher surgical risk candidates than those evaluated in the EVEREST II trial. More patients had functional MR, and the anatomical characteristics of the mitral valve in 70–80% of these patients were outside the inclusion criteria stated in the EVEREST II trial.33 Positive clinical outcomes with the Clip were still demonstrated at one year with improvements in degree of MR reduction and NYHA heart failure classification, QoL and six-minute-walk test results. Outside the USA, where Food and Drug Administration (FDA) approval has been limited to degenerative MR, and at least in Europe, this is currently the group of patients most commonly receiving MitraClip® repair.
Systematic reviews of MitraClip® versus surgery confirm that, while MitraClip® is not as effective as surgery in reducing MR, it can provide clinical benefits. Munkholm-Larsen et al.34 identified MitraClip® implantation as an option in managing select high surgical risk patients with severe MR, but noted a lack of mid- to long-term data in high-risk groups. In a meta-analysis by Wan et al.35 the clinical outcomes were similar despite a higher risk profile in the MitraClip® patients compared with surgical intervention, although surgery was more effective in reducing MR in the early post-procedure period. Buzzatti et al. reported over 5.5 years of a single centre’s experience comparing MitraClip® with surgery in octogenarians with degenerative MR.36 MitraClip® was safer, despite being applied in an older and more symptomatic population with higher burden of comorbidities. MR reduction was not as optimal as surgery, but provided reduction in symptoms. This improvement in QoL, with greater procedural safety and quicker recovery compared with surgery, as well as reduction in burden on health services, is of key relevance in managing the elderly population.
Guidelines
The European Society of Cardiology (ESC) guidelines for heart failure 201237 provide a class IIb (level of evidence C) indication for MitraClip® in patients with both degenerative and functional MR in order to improve symptoms. Patients must be judged inoperable or at unacceptably high surgical risk, and have a life-expectancy of greater than one year. Primary MR (degenerative MR) and secondary MR (FMR) reflect two very distinct clinical groups. Patients with FMR must be on optimal medical therapy including cardiac resynchronisation therapy where appropriate.
Functional MR – an emergent indication?
There remains some skepticism, particularly with respect to heart failure patients with FMR, due to the limited data from the EVEREST II RCT,30 and extrapolation from uncontrolled registry data.38 MR in heart failure confers a worse prognosis,39-41 but it has not been clear whether it is simply a marker of underlying LV muscle disease or a target for intervention.
Currently, four trials directly comparing MitraClip® to medical therapy in patients with heart failure and MR are underway to fill the current knowledge gap; one in the USA – COAPT (Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients Trial),42 and three in Europe – RESHAPE-HF (A Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation), MITRA-FR (Multi-centre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation) and MATTERHORN (A Multicenter, Randomised, Controlled Study to Assess Mitral vAlve reconsTrucTion for advancEd Insufficiency of Functional or iscHaemic ORigiN).43-45 These contemporary prospective, randomised trials will be instructive in guiding MitraClip® implantation in FMR, or indeed, mitral valve intervention of any kind.
Patient selection
A heart team comprised of cardiac surgeon, heart failure specialist, echocardiologist and interventional cardiologist is critical to determining the most appropriate patients for intervention with MitraClip® therapy.
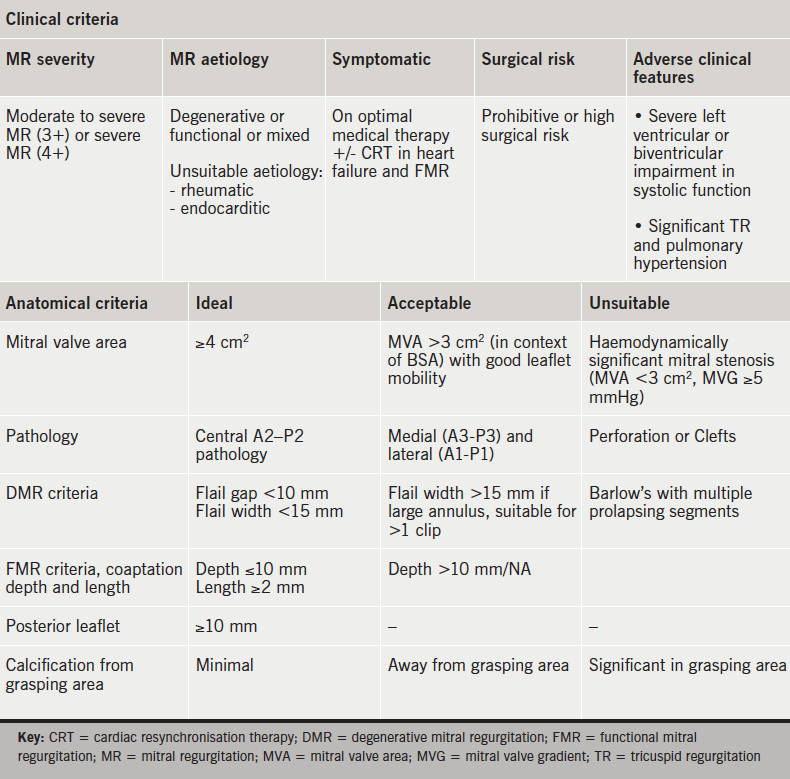
Patients are assessed for clinical indication, which broadly reflects the ESC guidelines.37 Evaluation may include stress echocardiography to assess for dynamic MR and cardiac magnetic resonance (CMR) imaging for viability and ischaemia testing. Technical suitability is determined by assessing the anatomical characteristics of the valve and pathology with TOE. Approximately 25% of patients referred for consideration of MitraClip® are considered suitable, which reflects the complex nature of MR. Moderate or severe tricuspid regurgitation and pulmonary hypertension are poor predictors of outcome with respect to NYHA functional class and re-hospitalisation with heart failure.
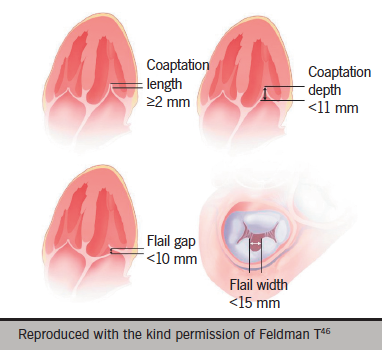
The EVEREST trial included stringent anatomical criteria that focused on the middle, A2–P2, segment of the valve.30 The anatomic eligibility criteria are shown in figure 2.46 The European experience suggests technical feasibility in a more complex group of patients can be achieved with an increase in the learning curve.32 Nevertheless, adverse anatomical features exist and recognition of these limitations is important to ensure optimal outcomes.
Table 2 provides a summary of the clinical and anatomical criteria.
MitraClip® in England
Although the technology has been commercially available in Europe since 2008, National Health Service (NHS) England only commissioned this technology for evaluation in 2014. The Commissioning through Evaluation (CtE) programme47 supports a small number of procedures to be funded within a limited number of selected centres and within a limited time frame. Three centres in the country were selected to deliver this therapy:
- Royal Brompton and Harefield Hospitals (RBHT), London
- University Hospital of Bristol (UHB)
- University Hospital of South Manchester (UHSM).
Each was commissioned to treat 40 patients per year. Associated data collection will provide evidence on the relative clinical- and cost-effectiveness of the procedure, which will be used to inform a commissioning policy decision. The MitraClip® registry is managed by NICOR (National Institute for Cardiovascular Outcomes Research), with the National Institute for Health and Care Excellence (NICE) supporting NHS England in the evaluation of the scheme.
Links to the three centres can be found below. These will direct you to dedicated proformas (which function as stand-alone referral letters or may be used to guide referral details) with coordinator contact details for the three centres:
- Royal Brompton and Harefield Hospitals, London
- University Hospital of Bristol
- University Hospital of South Manchester
Summary
MitraClip® offers a safe and effective percutaneous strategy for high surgical risk or inoperable patients. Comorbidity, such as frailty, severe lung disease or multi-organ decline, should be recognised as key limitations in achieving positive outcomes. Primary MR and secondary MR should be recognised as two distinct patient populations with specific approaches to diagnosis and treatment. Secondary MR reflects a ventricular problem that should primarily be managed via optimisation of medical therapy and cardiac resynchronisation therapy (CRT), where indicated. Registry data, however, suggest FMR as a target for intervention. RCTs will be crucial to informing whether this expansion in indication is supported for mitral valve therapies of any kind. The importance of timely intervention, before an irreversible decline in LV remodelling and pulmonary hypertension ensues, may focus attention on earlier intervention, if safe and effective outcomes are shown to be durable beyond five years.
Percutaneous strategies presently limit repair options to a single point of intervention. The MR reduction and clinical improvements observed with MitraClip® without annuloplasty may reflect the advantages of the vertical coaptation produced with the MitraClip®. Nevertheless, longer follow-up of patients with residual grade 2 MR in particular, will be important. Development of percutaneous direct annuloplasty technologies might offer the potential to enhance long-term outcomes.
Conclusion
Current transcatheter mitral valve techniques are at the beginning of an era of innovation before their full potential is realised. The broadening of available options for MR reduction is welcome and transcatheter mitral valve interventions provide complementary strategies in the drive for more safe and effective therapies for patients. Surgical repair will be the standard for many years, certainly in low risk DMR. Randomised trials that challenge and enhance our understanding of new technologies are vital, and will guide the risk-benefit analysis of different approaches. This will permit more tailored care according to individual patient profile. Multi-disciplinary and collaborative practice is essential to achieving optimal standard of care.
Funding
This article was commissioned by the British Journal of Cardiology on behalf of Abbott Laboratories Ltd. who provided the funding. BJC engaged Dr Mamta Buch, who received an honorarium for its writing. Abbott Laboratories had no input into the content of the article written by Dr Buch, but has reviewed the article for regulatory compliance before publication.
MitraClip® is a trademark of the Abbott Group of Companies. The product is subject to prior training requirement as per the Instructions for Use. The product is intended for use by or under the direction of an appropriately qualified medical practitioner. Prior to use, it is important to read the package insert thoroughly for instructions for use, warnings and potential complications associated with use of this device.
Conflict of interest
MHB has received a speaker’s honorarium from Abbott.
Key messages
- Mitral regurgitation (MR) is a complex and heterogeneous disease
- MitraClip® is a safe, less invasive option for patients with degenerative MR who are high risk or inoperable
- MitraClip® may provide benefits in patients with heart failure and severe functional MR who are symptomatic despite optimal medical therapy (including cardiac resynchronisation therapy)
- The NHS England MitraClip® Commissioning through Evaluation programme will determine whether patients in England will have access to MitraClip® therapy.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart disease: a population-based study. Lancet 2006;368:1005–11. http://dx.doi.org/10.1016/S0140-6736(06)69208-8
2. Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Diseasae. Eur Heart J 2003;24:1231–43. http://dx.doi.org/10.1016/S0195-668X(03)00201-X
3. Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–65. http://dx.doi.org/10.1093/eurheartj/ehm001
4. Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–43. http://dx.doi.org/10.1016/S0195-668X(03)00201-X
5. Lillehei CW. New ideas and their acceptance: as it has related to preservation of chordate tendinea and certain other discoveries. J Heart Valve Dis 1995;4(suppl2):S106–S114.
6. Perloff JK, Roberts WC. The mitral apparatus: functional anatomy of mitral regurgitation. Circulation 1972;46:227–39. http://dx.doi.org/10.1161/01.CIR.46.2.227
7. Otto CM. Clinical practice: evaluation and management of chronic mitral regurgitation. N Engl J Med 2001;345:740–6. http://dx.doi.org/10.1056/NEJMcp003331
8. Carpentier A. Cardiac valve surgery: the „French correction”. J Thorac Cardiovasc Surg 1983;86:323–37.
9. Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014;129:e521–e643. http://dx.doi.org/10.1161/CIR.0000000000000031
10. Vahanian A, Alfieri O, Andreotti F et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33:2451–96. http://dx.doi.org/10.1093/eurheartj/ehs109
11. Jokinen JJ, Hippelainen MJ, Pitkanen OA et al. Mitral valve replacement versus repair: propensity-adjusted survival and quality-of-life analysis. Ann Thorac Surg 2007;84:451–8. http://dx.doi.org/10.1016/j.athoracsur.2007.03.058
12. Enriquez-Sarano M, Schaff HV, Orszulak TA et al. Valve repair improves the outcome of surgery for mitral regurgitation: a multivariate analysis. Circulation 1995;91:1022–8. http://dx.doi.org/10.1161/01.CIR.91.4.1022
13. Horskotte D, Schulte HD, Bircks W et al. The effect of chordal preservation on late outcome after mitral valve replacement: a randomized study. J Heart Valve Dis 1993;2:150–8.
14. David TE, Uden DE, Strauss HD. The importance of the mitral apparatus in left ventricular function after correction of mitral regurgitation. Circulation 1983;68(suppl II):II-17–II-82.
15. De Bonis M, Lorusso R, Lapenna E et al. Similar long-term results of mitral valve repair for anterior compared with posterior leaflet prolapsed. J Thorac Cardiovasc Surg 2006;131:364–70. http://dx.doi.org/10.1016/j.jtcvs.2005.09.040
16. Gillinov AM, Cosgrove DM, Blackstone EH et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998;116:734–43. http://dx.doi.org/10.1016/S0022-5223(98)00450-4
17. Gillinov AM, Cosgrove DM. Mitral valve repair for degenerative disease. J Heart Valve Dis 2002;11(suppl 1):S15–S20.
18. Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381–7. http://dx.doi.org/10.1016/j.jacc.2004.09.073
19. Lee AP, Acker M, Kubo SH et al. Mechanisms of recurrent mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy. Circulation 2009;119;2606–14. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.796151
20. McGee EC, Gillinov AM, Blackstone EH et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916–24. http://dx.doi.org/10.1016/j.jtcvs.2004.07.037
21. Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–65. http://dx.doi.org/10.1093/eurheartj/ehm001
22. O’Brien SM, Shahian DM, Filardo G et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2 – isolated valve surgery. Ann Thorac Surg 2009;88(suppl 1):S23–S42. http://dx.doi.org/10.1016/j.athoracsur.2009.05.056
23. Webb JG, Harnek J, Munt BI et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 2006;113:851–5. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.591602
24. Leon MB, Smith CR, Mack M et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. http://dx.doi.org/10.1056/NEJMoa1008232
25. Quill JL, Hill AJ, Laske TG, Alfieri O, Iaizzo PA. Mitral leaflet anatomy revisited. J Thorac Cardiovasc Surg 2009;137:1077–81. http://dx.doi.org/10.1016/j.jtcvs.2008.10.008
26. Alfieri O, Maisano F, De Bonis M et al. The double-orifice technique in mitral valve repair. A simple solution for complex problem. J Thorac Cardiovasc Surg 2001;122:674–81. http://dx.doi.org/10.1067/mtc.2001.117277
27. De Bonis M, Lapenna E, La Canna G et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the “edge-to-edge” technique. Circulation 2005;112(9 suppl):I402–I408.
28. St Goar FG, Fann JI, Komtebedde J et al. Endovascular edge-to-edge mitral valve repair: short-term results in a porcine model. Circulation 2003;108:1990–3. http://dx.doi.org/10.1161/01.CIR.0000096052.78331.CA
29. Herrmann HC, Feldman T. Percutaneous mitral valve edge-to-edge repair with the Evalve Mitraclip system: rationale and phase I results. EuroIntervention 2006;1(suppl A):A36–A39.
30. Feldman T, Foster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–1406. http://dx.doi.org/10.1056/NEJMoa1009355
31. Feldman T, Kar S, Elmirah S, et al. Randomised comparison of percutaneous repair and surgery for mitral regurgitation. J Am Coll Cardiol 2015;66:2844–54. http://dx.doi.org/10.1016/j.jacc.2015.10.018
32. Maisano F, Franzen O, Baldus S et al. Percutaneous mitral valve interventions in the real world. Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip® therapy in Europe. J Am Coll Cardiol 2013;62:1052–61. http://dx.doi.org/10.1016/j.jacc.2013.02.094
33. Schillinger W, Athanasiou T, Weicken N et al. Impact of the learning curve on outcomes after percutaneous mitral valve repair with MitraClip® and lessons learned after the first 75 consecutive patients. Eur J Heart Fail 2011;13:1331–9. http://dx.doi.org/10.1093/eurjhf/hfr141
34. Munkholm-Larsen S, Wan B, Tian DH et al. A systematic review on the safety and efficacy of percutaneous edge-to-edge mitral valve repair with the MitraClip system for high surgical risk candidates. Heart 2014;100:473–8. http://dx.doi.org/10.1136/heartjnl-2013-304049
35. Wan B, Rahnavardi M, Tian DH et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann Cardiothorac Surg 2013;2:683–92. http://dx.doi.org/10.3978/j.issn.2225-319X.2013.11.02
36. Buzzatti N, Maisano F, Latib A et al. Comparison of outcomes of percutaneous MitraClip versus surgical repair or replacement for degenerative mitral regurgitation in octogenarians. Am J Cardiol 2015;115:487–92. http://dx.doi.org/10.1016/j.amjcard.2014.11.031
37. McMurray JJ, Adamopoulos S, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. http://dx.doi.org/10.1093/eurheartj/ehs104
38. Coats AJ, Shewan LG. Inconsistencies in the development of the ESC Clinical Practice Guidelines for Heart Failure. Int J Cardiol 2013;168:1724–7. http://dx.doi.org/10.1016/j.ijcard.2013.05.045
39. Junker A, Thayssen P, Nielsen B et al. The hemodynamic and prognostic significance of echo-Doppler-proven mitral regurgitation in patients with dilated cardiomyopathy. Cardiology 1993;83:14–20. http://dx.doi.org/10.1159/000175942
40. Conti JB, Mills RM Jr. Mitral regurgitation and death while awaiting cardiac transplantation. Am J Cardiol 1993;71:617–18. http://dx.doi.org/10.1016/0002-9149(93)90526-I
41. Blondheim DS, Jacobs LE, Kotler MN et al. Dilated cardiomyopathy with mitral regurgitation: decreased survival despite a low frequency of left ventricular thrombus. Am Heart J 1991;122:763–71. http://dx.doi.org/10.1016/0002-8703(91)90523-K
42. Clinicaltrials.gov. Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy For High Surgical Risk Patients (COAPT). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01626079
43. Clinicaltrials.gov. A Randomised Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Mitral Regurgitation (RESHAPE-HF). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01772108
44. Clinicaltrials.gov. Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01920698
45. Clinicaltrials.gov. A Multicenter, Randomized, Controlled Study to Assess Mitral vAlve reconsTrucTion for advancEd Insufficiency of Functional or iscHemic ORigiN (MATTERHORN). Available at: http://www.clinicaltrials.gov/ct2/show/NCT02371512
46. Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686–94. http://dx.doi.org.10.1016/j.jacc.2009.03.077
47. NHS England. Commissioning through Evaluation. Available at: https://www.england.nhs.uk/commissioning/spec-services/npc-crg/comm-eval/
