Correspondence from the world of cardiology
Dear Sirs,
Acute decompensated heart failure (HF) is the most common cause of hospital admission among patients older than 65 years of age.1 Such patients present with dyspnoea and variable degrees of fluid retention. Although aldosterone is known to be elevated in patients with HF, it is not yet established whether aldosterone levels affect clinical presentation. We have performed a preliminary study to investigate the degree of variation in baseline aldosterone and whether there is any relationship between aldosterone levels and the extent of peripheral oedema.
Methods and results
We enrolled 29 patients (mean age: 76 years; range: 43−90) admitted to the cardiology ward with a diagnosis of acutely decompensated chronic HF (based on Framingham criteria).2 Following informed consent, blood samples for aldosterone levels were taken on the first morning after admission with the patient recumbent. Serum creatinine and brain natriuretic peptide (BNP) were measured simultaneously to control for renal function (GFR) and severity of HF respectively.3 The extent of oedema was assessed using a standard scoring system (see table 1) and patients were assessed clinically for the presence of ascites.

All patients had moderate or severe impairment of left ventricular function and were either New York Heart Association (NYHA) class 2 (n=8) or 3 (n=21). The majority had ischaemic heart disease (n=18) and were taking beta blockers (n=19), angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) (n=25) and mineralocorticoid receptor antagonists (MRAs) (n=18). Aldosterone levels varied widely (mean: 496; range 60–2775 pmol/dl) and there was no correlation between aldosterone and BNP (Pearson’s correlation coefficient r² = 0.02) or between aldosterone and GFR (r² = 0.13).
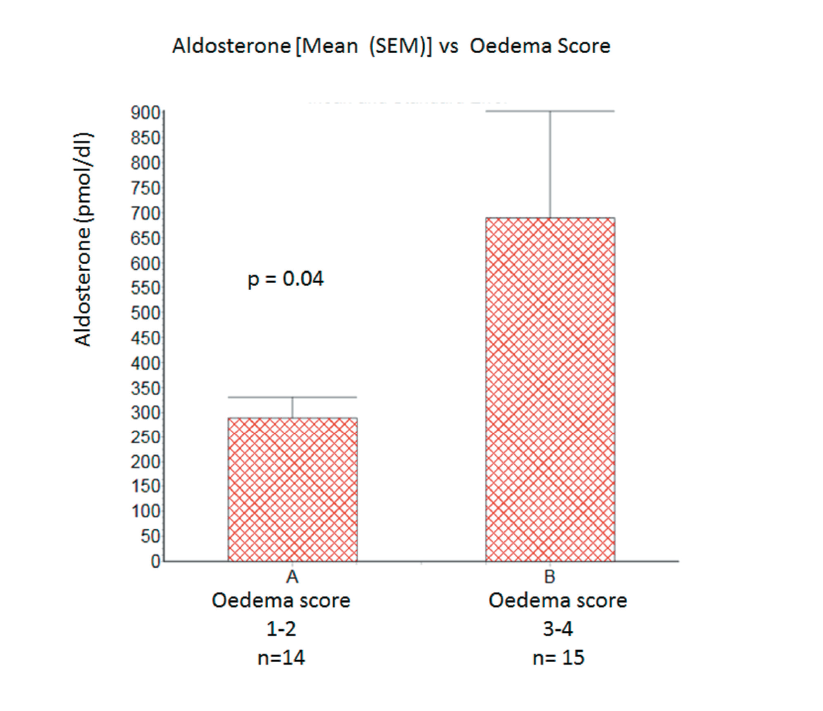
Aldosterone levels were significantly higher in patients with oedema score 3-4 than in those with oedema score 1−2 [mean (SEM): 688 (214) vs. 289 (41) pmol/dl; p=0.04 one-tailed unpaired t-test]. See figure 1. Aldosterone levels were highest in patients (n=5) with oedema score 3−4 together with clinically detectable ascites [mean (SEM): 1019 (394) pmol/dl].
Conclusion
The results of this preliminary investigation suggest that aldosterone levels vary widely in patents presenting with acutely decompensated HF but appear to be highest in those with gross peripheral oedema. This relationship appears to be independent of renal function and overall severity of HF. Our data also suggest that aldosterone is particularly elevated in patients with ascites though the small numbers preclude meaningful statistical analysis.
Although oedema formation in HF is a complex and multifactorial process, our preliminary data suggest that aldosterone elevation may play a pivotal role in its pathophysiology. In this regard a comparison may be made with advanced cirrhotic liver disease, a condition in which aldosterone levels are also elevated in conjunction with ascites formation and peripheral oedema. Although the pathophysiology is similarly complex, impaired hepatic metabolism of aldosterone is a key factor, in addition to stimulation of the renin angiotensin axis in response to reduced circulating volume.4 It may be hypothesised that similar mechanisms apply in heart failure with hepatic function compromised by congestion and chronic ischaemia.
The comparison may have therapeutic implications in that aldosterone antagonists (MRAs) are prescribed at substantially higher doses for cirrhotic liver disease than for congestive cardiac failure.5 In contrast, loop diuretics are the mainstay of treatment for heart failure with MRAs added at relatively low doses.6–8 We suggest that a more definitive study should be undertaken in a larger cohort of patients. If our initial findings are confirmed, further studies investigating the targeted use of high dose MRAs may be justified in patients with severe oedema and/or ascites.
Acknowledgements
Dr Paul Sainsbury for refereeing the ethics submission and Dr Lindsey Yeoman for statistical analysis.
Conflict of interest
None declared.
Gareth Archer
Specialist Registrar in Cardiology
Stephanie Hughes
Specialist Registrar in Cardiology
Haqeel Jamil
Specialist Registrar in Cardiology
Edward Bounford
Senior House Officer in Cardiology
Robert Stevenson
Consultant Cardiologist
Department of Cardiology, Huddersfield Royal Infirmary, Huddersfield
References
1. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. J Am Med Assoc 2005;293:572e80.
2. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22(4 Suppl A):6A−13A.
3. Hobbs R. Using BNP to diagnose, manage and treat heart failure. Cleveland Clinic J Med 2003;70:333−6.
4. Bansal S, Lindenfeld JA, Schrier RW. Sodium retention in heart failure and cirrhosis: potential role of natriuretic doses of mineralocorticoid antagonist? Circulation Heart Failure 2009;2:370−6.
5. Bernardi M. Optimum use of diuretics in managing ascites in patients with cirrhosis. Gut 2010;59:10−11.
6. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797−805.
7. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709−17.
8. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309−21.
