The innovative and trustworthy use of routinely collected healthcare data has enabled a UK company, NorthWest Ehealth, experts in the use of this technology, to become the only organisation in the world to have evaluated the safety and effectiveness of a pre-license medicine in a real-world setting. Its technology can support the whole clinical trial lifecycle and enable more effective feasibility, economic modelling, recruitment, real-time safety monitoring and data analytics.
‘The use of electronic healthcare records for clinical research is helping change the clinical trial landscape’
The EHR and future of clinical trials
The use of electronic healthcare records (EHRs) for clinical research is helping change the clinical trial landscape to be more relevant to everyday practice. Use of the EHR can help in the development of study design and to better understand the trial population and follow-up. The potential data set extends well beyond the period of the clinical trial, which allows a much longer-term assessment of the evolution of chronic conditions and the impact of interventions.
Salford Lung Studies
The Salford Lung Studies have demonstrated how EHRs can be used to support clinical trials. Salford is a community served mainly by a single hospital with an established EHR system that connects primary and secondary care. This setting allowed patients to be observed unobtrusively for effectiveness and safety monitoring, blended into routine clinical care.
The first Salford Lung Study was designed to evaluate the effectiveness and safety of the once-daily inhaled combination of fluticasone furoate and vilanterol (fluticasone furoate–vilanterol) as compared with existing maintenance therapy (usual care) in a large, real-world population of patients with chronic obstructive pulmonary disease (COPD) in conditions of normal care.1 It was a prospective, 12-month, open-label, parallel-group, randomised trial conducted in 77 general practices in Salford and South Manchester. The second Salford Lung Study assessed the same product in asthma with a similar study design.2 Over 7,200 patients were monitored in near real-time for safety and outcomes using city-wide linked EHRs.
Utilising data from EHRs helps:
- understand the patient pathway and disease before, during and following the trial programme
- provide the basis for richer analysis and deeper understanding of care pathways and disease progression
- confer many advantages over traditional study methods, such as the availability of real-time safety monitoring. The potential for drug safety information to be monitored more rapidly could allow faster decisions regarding the continuation/cessation of trials in the interests of patient safety.
What happened first?
- In the Salford Lung Studies, NorthWest Ehealth worked in partnership with the study sponsor GSK, and learned together as they progressed through the studies. There was a steep learning curve as this was the first time a study had been conducted in this way.
- The mechanisms that needed to be put into place and considerations included:
- study design – how could internal and external validity be best achieved?
- feasibility – what data existed? How good were the data? How could it be accessed, managed and validated? What were the needs of research authorities, regulators and payers? Was there a large enough population to power such a study?
- getting large numbers of people on board including:
- practitioners – GPs, pharmacists, specialists
- regulators and payers
- hospital managers, chief information officers, pharmacy chains/providers
- the creation of a research delivery team, with staff trained to good clinical practice (GCP) standards
- building a fully validated system and dataset, which needed the following challenges to be overcome:
- devising processes for managing the data flows
- building a safety reporting application and processes, and a community pharmacy data collection
- delivering data in the sponsor-determined format
- designing and delivering a data archival solution.
Study design
Following screening of medical records, patients were asked it they would like to participate in the study and gave written informed consent if they met the inclusion and exclusion criteria. They were randomly assigned to receive the study inhaler or to continue their usual maintenance therapy within the following two months.
The use of existing EHR data was maximised to fulfil protocol and analysis requirements, including total cost of care, care pathways and adherence. The key investigators in the trial were general practitioners, who could choose appropriate therapy according to their clinical opinion. Treatments were dispensed by community pharmacies in the usual way. Patients could switch from the study treatment to usual care, but patients in the usual-care group were not permitted to switch to the study inhaler group.
Importantly, the trial was designed in collaboration with regulators and payers. To preserve the real-world nature of the trial, the patients’ experience was as close to everyday clinical practice care as possible. Contact with trial staff was minimised. If at months 3, 6, and 9, patients had not had any contact with their general practice within the previous eight weeks, then they were contacted by telephone by a trial team member to assess any serious adverse events or non-serious adverse drug reactions; there was no additional intervention at these assessments. At 12 months, trial staff met the patients to make a final assessment of outcomes. Thus, most patients had contact with trial staff only at recruitment, at the baseline visit, and at 12 months.
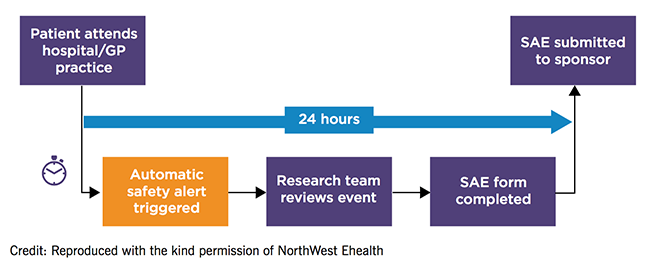
The safety alerting and reporting system in the Salford Lung Studies
Study delivery
The amount of manually entered data was minimised with primary and secondary care connected through the EHR. This was integrated with a new data recording system, ConneXon, that enabled the collection of a trial-relevant dataset with the highest standards of data management and validation – more than three million lines of data were collected for all the effectiveness and safety outcomes.
Patients were monitored remotely with the use of the EHR for the early detection of safety events. This system was adjusted to ensure that the right amount of data was transmitted to the authorities, as initially the wealth of ‘real-time’ data that could be collected regarding adverse events was overwhelming. A time-delayed submission of adverse event reports was decided upon in order to allow the full resolution and investigation of adverse events to be included in one report.
Conclusion
The Salford Lung Studies challenge the automatic transfer of findings from efficacy studies to clinical guidelines or everyday clinical practice. For any new treatment, it is essential to carry out safety and efficacy randomised, controlled trials. But these are carried out in carefully selected populations, which exclude patients with co-existing conditions. In addition, the frequent face-to-face monitoring seen in randomised, controlled trials ensures high adherence to therapy. Trials conducted using EHRs can be conducted in a largely unsupervised population, which allows important factors in usual clinical care, such as adherence, frequency of dosing, and persistence of good inhaler technique, to come into play.
Challenges for scaling
- The Salford Lung Studies took place in a single urban area and relied on the existence of a connected community of care. Expanding geographically requires:
- Connection of people and technology across the region of study.
- Consistency of data standards.
- A study data tabulation model (SDTM) to provide a standard for organising and formatting data to streamline processes in collection, management, analysis and reporting.
- Data need to be available at the required level of quality.
- Solutions to extract data.
- Regulatory support for change.
Lessons learned from the Salford Lung Studies
- Innovation can be disruptive.
- Engage early with all the study teams to manage expectations.
- New ways of working are a learning experience for the sponsor organisation.
- Anticipate the impact of factors, such as statistics, on the existing sponsor internal teams – more data are not always the answer. The right data are needed. There can be too much data.
- Only collect what is needed from outside the electronic medical record – potential transcription errors require source data verification requirements, which can severely impact efficiency when this is done on a big scale.
Summary
- The use of EHRs in clinical studies could accelerate the progression of new treatments through the efficacy and effectiveness requirements of regulatory authorities.
- Novel treatments could be made available in everyday practice sooner.
- Enhanced drug safety data with much longer-term follow-up would be collected.
- Patients would be involved in trials with minimal impact as healthcare would be accessed normally via their regular healthcare professionals.
Articles in the handbook
1. Introduction
2. National Institute for Health Research
4. Recent research at the Institute of Cardiovascular Medicine and Science
5. Clinical trials in the UK from a commercial perspective
6. Brexit: threat or opportunity
7. How to initiate a clinical trial in the UK
8. Useful organisations
References
1. Driessen MT, Barnfather S, Mulley PJ, Boucot II, Ignacio T, van de Wetering G. The cost-consequence of fluticasone furoate/vilanterol 100/25 mcg in the UK using the results from the COPD Salford Lung Study. Thorax 2016; 71(suppl 3). http://dx.doi.org/10.1136/thoraxjnl-2016-209333.200
2. Woodcock A, Vestbo J, Bakerly ND et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 2017;390:2247–55. https://doi.org/10.1016/S0140-6736(17)32397-8