This investment in research also benefits the UK economy providing employment, trade, and addressing shortfalls in STEM skills (Science, Technology, Engineering, and Mathematics). In addition to giving patients faster access to treatments, getting efficacious and safe medicines registered more efficiently enables the trial sponsor to gain reimbursement for those medications sooner.
Evolving bio-pharmaceutical model
Medical research changed dramatically with the discovery of the structure of DNA and the subsequent understanding of genetic code, with DNA sequencing and amplification. This has led to medicines becoming increasingly personalised leading to improved outcomes. Disease can now be predicted through the identification of predisposition markers or underlying processes, allowing for targeted prevention.
Early identification of disease using low-cost stratification/marker tests can lower the costs of disease management. In some cases, diseases can be detected years before the onset of symptoms, which has allowed earlier intervention to prevent morbidity, often with relatively low-cost treatments.
Precise diagnoses – based on underlying cause rather than on grouped symptoms – have been facilitated by rapid turnaround diagnostics, near-time analysis and interpretation. Identification of effective treatments enables more targeted intervention and an increasingly evidence-based course of action can be taken with a personalised treatment plan. With the ability to assess predisposition to drug response, subpopulation therapy becomes more economic.
All this has the potential to dramatically improve outcomes:
- conditions can be identified and managed at an early stage
- therapies are only used when indicated, with fewer unwanted side effects and adverse events
- patient experience is improved through more participation
- costs are reduced with greater efficiency from streamlined care pathways, earlier and more precise diagnosis and treatment, fewer and less complicated surgical interventions, and fewer patients getting cancer and other diseases.
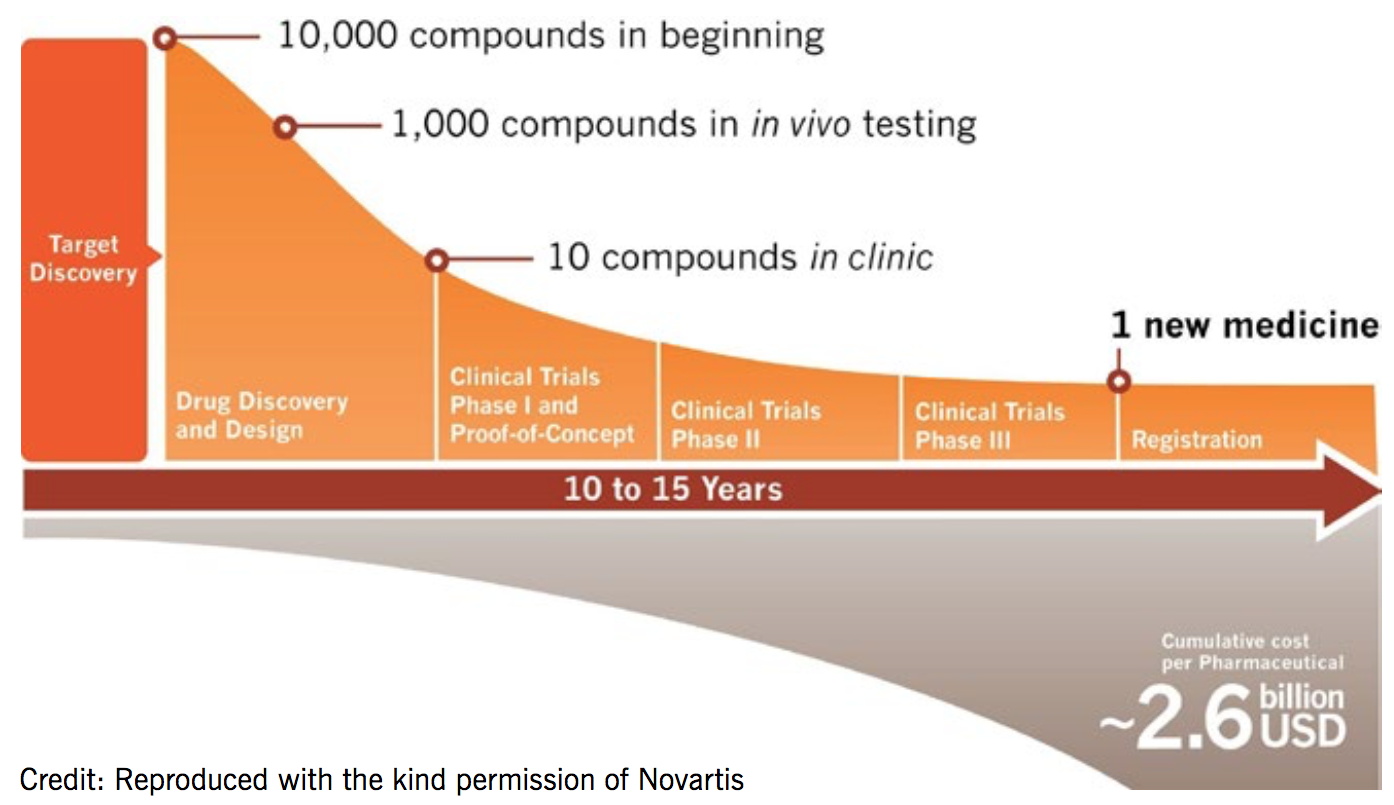
Drug development takes an average of 14 years and costs US$2.6 billion for each new medicine
‘Medicines are becoming increasingly personalised leading to improved outcomes’
The Novartis approach to research
Novartis has a large product pipeline with over 90 new molecular entities in the clinic. Its innovative research has produced 13 US Food and Drug Administration (FDA) breakthrough therapy designations in the last five years. In 2016, Novartis had seven FDA fast-track designations, and a good success rate, with 29 approvals in the last five years.
In the UK, Novartis was the leading industry-led trial sponsor for phase II–IV studies in 2014–2016. This has enabled Novartis to conduct clinical research at over 850 sites across the UK.
Novartis Institutes for BioMedical Research
The Novartis Institutes for BioMedical Research (NIBR) are the research arm of Novartis, and bring medicines rapidly into the clinic. Their focus is on tractable biological targets with an excellent rationale for addressing unmet medical need. NIBR’s mission is driven by a patient-centric approach, with increasing access to the genomic and chemical universe providing new methods for drug discovery.
NIBR employs about 6,000 scientists at seven global sites. About 90 new molecular entities are currently being developed across prevalent disease areas, with about 400 active research projects and over 500 ongoing clinical trials.
Its drug development process is iterative with data and information flowing in and out of all stages in multiple directions. Resources are applied to projects to support the answering of key scientific questions. Projects can initiate or terminate at any point. NIBR handles research up to the point of phase IIb clinical trials, at which point the novel molecular entity (NME) is transitioned to the global drug development team. It is estimated to take an average of 14 years and US $2.6 billion to get a single drug to market.
Summary
- The UK currently has a leading role in healthcare research, and the industry/academia/healthcare partnership is key.
- The objective of outcomes-focused healthcare systems is to deliver better patient outcomes at the same or lower cost. This relies on quality outcome data as a starting point to improve the care cycle.
- There will always be a role for traditional discovery, translational and phase III studies.
- Outcomes-based sustainable healthcare systems need new approaches to use of data.
Articles in the handbook
1. Introduction
2. National Institute for Health Research
3. Optimising clinical research using electronic medical records
4. Recent research at the Institute of Cardiovascular Medicine and Science
6. Brexit: threat or opportunity
7. How to initiate a clinical trial in the UK
8. Useful organisations