‘The NHS has indicated that research is a clear priority in improving healthcare for patients’
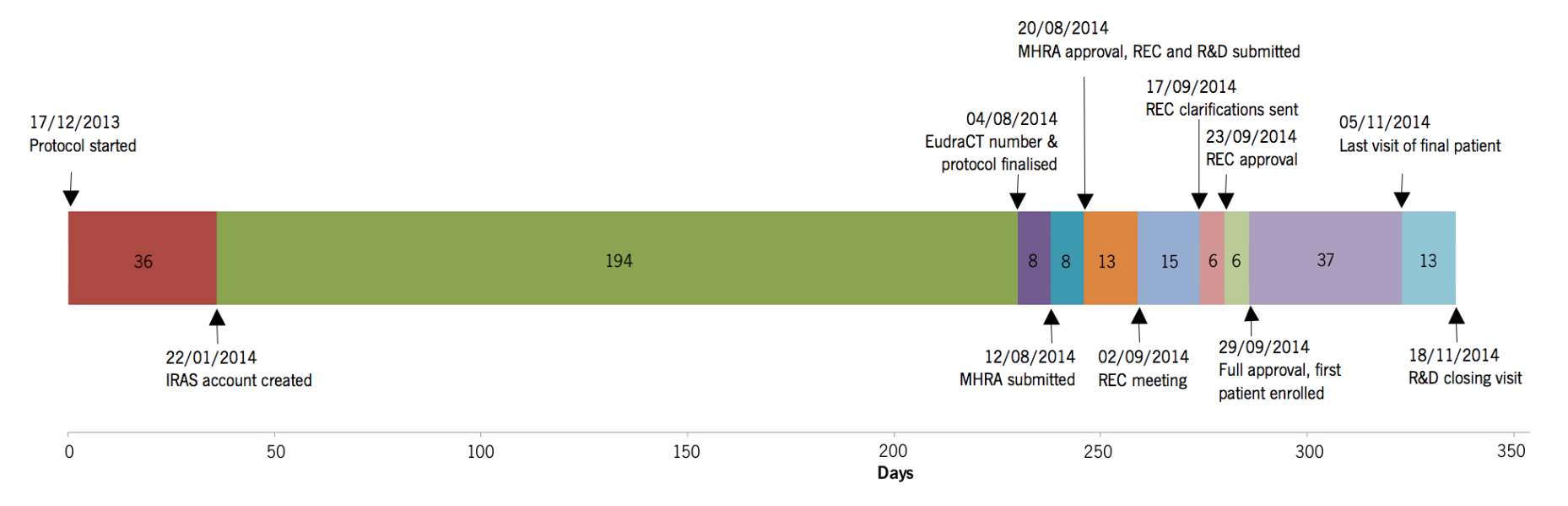
Figure 1. The case trial timeline, intervals are in days
A summary of the article’s key learning points
- Obtaining approval for conducting clinical research can be an extremely lengthy endeavor (see figure below). The process makes a four-month term in research almost useless, unless you begin the application process at least nine months in advance of a trial’s expected start date. You will need more than a year if you are applying for external funding.
- Work closely with the relevant organisations, particularly the R&D department of your Trust, the Research and Ethics Committee and the Medicines Health Research Agency to reduce the risk of avoidable errors and delay.
- A robust study protocol is essential for a successful application and trial.
- Be guided by your academic supervisor: his/her advice is invaluable.
- Complete the online or face-to-face Good Clinical Practice (GCP) course offered by the National Institute of Health Research. It improves your understanding of clinical research. Successful completion of a GCP course is mandatory for those conducting clinical trials.
- Be ready to learn. You will acquire many skills during the application process: patience and endurance; effective communication and time management; critical appraisal; appreciation of ethical issues and patient safety.
- To be successful, the investigators have to communicate their capacity to manage potential complications, complete the trial and present the results of a relevant, well-designed study.
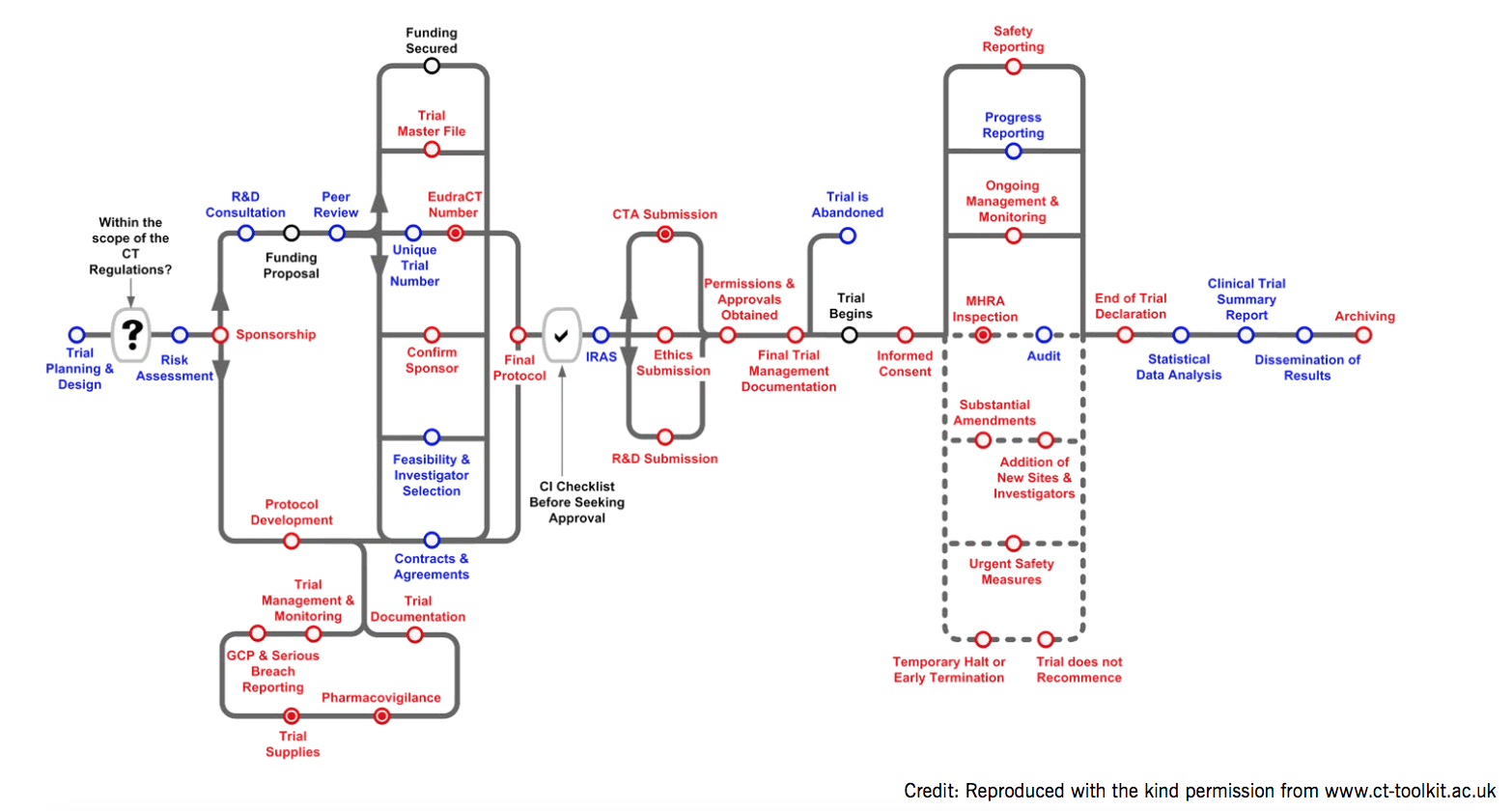
Figure 2. The complexity of a clinical trial: good practice requirements in blue and legal requirements in red. An informative pathway found on www.ct-toolkit.ac.uk/routemap
Articles in the handbook
1. Introduction
2. National Institute for Health Research
3. Optimising clinical research using electronic medical records
4. Recent research at the Institute of Cardiovascular Medicine and Science
5. Clinical trials in the UK from a commercial perspective
6. Brexit: threat or opportunity
8. Useful organisations