
Introduction
2018 marks the 60-year anniversary of the first patient to receive an implantable battery-powered pacemaker.1 In fact, the earliest recorded description of pacing was in 1882, and now countless lives have been saved by pacemaker technology. Subsequent important developments include the development of the implantable cardioverter defibrillator (ICD) in 1980 and then, more recently, one of the most effective cardiac treatments, cardiac resynchronisation therapy, which was first described in 1984.
Exciting and disruptive developments in pacing technology may, potentially, herald a sea change in practice, while historical findings are returning to reshape pacing. At this important moment in the history of cardiology, this article describes recent practice and likely future developments, including the rediscovery of His-bundle pacing.

Indications for pacing
Pacemakers are indicated for symptomatic bradycardia and also, in the case of atrio-ventricular (AV) block, prognosis. Largely, cardiac resynchronisation pacemakers are indicated in patients who have developed significant intraventricular conduction delay or those with left ventricular (LV) impairment who are expected to receive a high burden of right ventricular (RV) pacing. RV pacing is associated with the development of LV impairment, and it has been shown to increase mortality in those with pre-existing LV impairment. Approximately 10–20% of patients with normal LV function will develop LV impairment following significant (>40% paced beats) RV pacing. ICDs were initially introduced for survivors of sudden cardiac death, yet, it became clear that high-risk patients could be identified prior to potentially lethal ventricular arrhythmias. Nowadays, the majority of ICDs are implanted in patients for primary prevention in those who are judged to be a high risk of ventricular arrhythmias.
Developments in implantation techniques
The change in doctors training has limited the amount of surgical exposure prior to embarking on a career in cardiology. As a result, surgical skills training at the Royal College of Surgeons, regarded as an entry condition prior to surgical training, has been introduced for cardiology trainees and has allowed cross-fertilisation with modern surgical techniques. Trainee doctors, and cardiac physiologists are encouraged to achieve device specific accreditation, endorsed by professional bodies through examination and experience. Perhaps all this is a reflection of a maturing technology, increasing sub-specialisation alongside, rightfully, a continuous improvement culture to ensure patient safety. It is sobering to reflect that one in six patients will experience a device-related complication by three years.2
Prior to embarking on implantation, a decision needs to be made regarding anticoagulation. Current guidance is to avoid interrupting warfarin by bridging patients with heparin, but to continue warfarin-based anticoagulants in those at high thrombosis risk. Patients under the age of 75 do not experience a greater risk of haematoma with continued direct oral anticoagulants when coupled with the use of surgical diathermy to control bleeding.3 Control of bleeding is essential, as haematoma formation is a significant risk factor for subsequent device infection. For those patients undergoing box changes and extraction, the use of a plasma blade adds to the safety and reduces the time of procedures. Adrenaline-soaked gauze is often helpful in reducing bleeding from antiplatelet agents.
Modern surgical preparation with a single pre-implant dose of intravenous antibiotics, chloroprep (in place of iodine) and iodine-based skin adhesive drapes are used widely to minimise the risk of infection. The use of adrenaline with local anaesthetic is associated with improved haemostasis, and patient comfort can be increased by mixing with 1–2 ml 8.4% sodium bicarbonate.4 During generator changes it is worth noting that the 2017 Heart Rhythm Society guidelines advise against capsule removal during box changes because of the increased risk of haematoma and subsequent infection. Inert synthetic braided sutures, such as Ti-Cron and Ethibond, have replaced silk for securing lead sleeves, due to the significantly lower risk of infection. Wound closure with antibiotic impregnated sutures, e.g. Vicyl plus, are now recommended, alongside monofilament synthetic sutures, such as Biosyn. Other techniques such as fibrin sealants are available to reduce the risk of pocket haematoma. Traditionally, pressure bandages have been applied to minimise haematoma, and this has been refined with inflatable pressure gauze available, which can be applied rather like a radial artery pressure band.
Remote follow-up
The purpose of the traditional pacemaker clinic visit is being re-evaluated due to the capacity of devices to send data remotely. Modern devices are capable, and, to a large extent, function autonomously requiring minimal adjustment. Automatic threshold determination minimises battery usage. In fact, only 10% of patients require reprogramming during routine visits, and, hence, much follow-up activity can potentially be conducted remotely. Furthermore, the need for paper printouts has largely vanished with ubiquitous portable document formats available, and this may be coupled with pacemaker programmer databases, which remove the need for manual data input using systems such as Fysicon (note Phillips do not currently support Fysicon).
During follow-up, device-detected atrial fibrillation is common, and may not be detectable clinically in those who are ventricular pacing. Continuous episodes lasting greater than 24 hours benefit from risk assessment and anticoagulation, where appropriate.5
Magnetic resonance imaging (MRI) in patients with devices
Until the last decade, an implanted device was regarded as a contraindication to MRI scanning. This led to the development of MRI conditional devices, which allowed MRI scans in certain body areas as long as specific criteria were met. Meanwhile, large registry studies have examined the risk of scanning all patients with devices, and largely there is not an issue in patients who have defibrillation deactivated and are programmed to asynchronous pacing.6
Developments in bradycardia pacing
There is a major ongoing demand for bradycardia pacing, with an ageing and increasing population. Iterative improvements in standard pacemaker technology continue, but without major new developments. An important focus remains on minimising RV pacing with the recognition that even patients presenting with complete AV block may have periods of normal conduction. The reduction in RV pacing should be balanced with the avoidance of excessive PR intervals (>350 ms). A recent innovation has been leadless wireless pacemakers implanted into the RV apex. A Boston Scientific leadless device will be able to provide anti-tachycardia pacing communicating wirelessly with a subcutaneous defibrillator. Potentially, multi-site wireless pacing could prove attractive compared with current practice.
Developments in ICDs
In terms of indications, recent Danish work cast into doubt the need for defibrillator backup in patients with dilated cardiomyopathy (DCM), although a subsequent meta-analysis reports a similar benefit to those with ischaemic cardiomyopathy.7 Potentially higher risk DCM patients may be detected using cardiac MRI (CMR) to seek mid-wall fibrosis, a risk factor for sudden cardiac death. The absence of mid-wall fibrosis will allow identification of those at lower risk of sudden cardiac death.
Improvements in battery technology have dramatically reduced the need for box changes, as well as reducing box size. At the time of box change, reassessment of the benefit of ICD therapy is recommended, particularly in those who have never required therapy, who have significant frailty or worsening heart failure.

There is a choice of subcutaneous or transvenous ICDs. Most patients continue to receive transvenous systems, which offer ATP (anti-tachy pacing), with modern practice largely using single coil (RV coil) rather than dual coil (superior vena-cava [SVC] and RV) due to the similar effectiveness at defibrillation and perceived reduced risk of SVC injury during extraction with a single coil. Subcutaneous defibrillators have been shown to be safe, eliminate the need for transvenous leads and, due to the large electrocardiogram (ECG) vector, are highly effective at discriminating between ventricular tachycardia and other rhythms, but currently do not offer ATP. Defibrillation safety margin (DSM) testing at the time of implantation was routine, but has largely been discontinued based on the SAFE-ICD trial (Safety of Two Strategies of ICD Management at Implantation), which demonstrated an absence of benefit and a low risk of harm in patients during DSM testing.8 Practice varies, but generally DSM testing is still undertaken in patients judged to be likely to have high DSMs, such as obese patients, abnormal shock impedances, right-sided implants and subcutaneous ICD (SCD) implants.
Programming ICDs has changed due to clinical trials showing that longer detection intervals prior to initiating anti-tachycardia pacing (ATP), combined with higher ventricular rate cut-offs, have significantly reduced both appropriate and inappropriate (e.g. atrial fibrillation) therapy which, importantly, is associated with reduced mortality.
Cardiac resynchronisation therapy (CRT)
Since the first description of CRT 24 years ago, CRT has been shown to be one of the most effective cardiovascular therapies in terms of absolute reductions in mortality and heart failure (HF) hospitalisation. Clinical trials have demonstrated that the key to selecting patients who will benefit is the presence of electrical conduction delay alongside significant LV systolic impairment (ejection fraction [EF] ≤35%) in patients receiving optimal tolerated medical therapy. A critical determinant of failure to benefit is the presence of posterior-lateral scar demonstrated by CMR, particularly when the LV pacing lead is placed on transmural scar.9
CRT should be considered for an additional group of patients who are expected to have a high burden of RV pacing, and who have LV impairment, which is likely to worsen with RV apical pacing.
The main focus is ensuring good resynchronisation with well-placed, effective leads, and sufficient well-programmed biventricular pacing. The technology underpinning CRT has been refined, including delivery systems, but without doubt, the major leap has been in lead technology, with routine use of quadripolar LV pacing leads due to their associated reduction in phrenic nerve pacing, re-interventions and mortality.10 The major benefit of quadripolar over bipolar lead technology is the ability to programme around loss of LV lead capture. Even brief interruptions in CRT, such as awaiting outpatient lead repositioning, are associated with HF admission and mortality.11 Ensuring ≥92% of heart beats are from CRT is vital, and patients with frequent ectopics or atrial fibrillation limiting CRT delivery require attention. The latter group benefit most from AV junction ablation rather than rate-slowing medication alone.
Recent innovations in pacing the LV, such as LV-only pacing using the intrinsic conducting pathway by sensing off the RV lead and then LV only pacing, are attractive and appear beneficial. Ongoing investigations into multi-point LV pacing (pacing two poles of the LV lead sequentially) will determine whether these have clinical impact. The current paradigm is to place the LV lead in a posterior-lateral vein with proximal pole pacing to avoid LV apical pacing. Elegant studies have demonstrated the benefit of wide electrical separation between the ventricular leads, ensuring a sufficient time delay between RV and LV sensed QRS, ideally with the sensed LV electrogram in the latter third of the QRS.
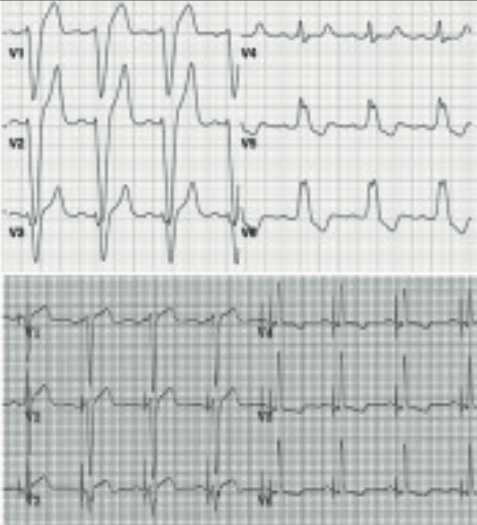
His-bundle pacing (HBP) and direct left bundle branch pacing
Perhaps the most exciting recent development in cardiac pacing is His-bundle pacing (HBP), which has the potential to disrupt current practice away from RV pacing. The benefits are immediately apparent using the natural conduction system to replicate the natural electrical conduction, resulting in a narrow QRS morphology similar to the intrinsic conduction. One major benefit is the reversal of left-bundle branch block (LBBB) by His-bundle pacing, which was first described 40 years ago. His-bundle pacing (HBP) has been described as “HBP has it all: the beauty of basic electrophysiology, the elegance of simplicity, the parsimony of cost-conscious care, and the joy of helping people”.12 Enthusiasts discuss the technique on Twitter #dontdisthehis. Small trials have shown a significant benefit in terms of reduction of heart failure, atrial fibrillation and mortality in those receiving >40% RV pacing.13
Standard pacemakers can be programmed to HBP, and a standard lead and appropriate guide are available. During implantation, at our unit we use a slave monitor to the pacing system analyser, allowing visualisation of the His-bundle electrogram prior to lead deployment. HBP often requires slightly higher lead outputs, and some standard pacing algorithms such as ventricular safety pacing should be switched off with HBP. In view of the benefits seen in small studies, HBP merits randomised-controlled trials to move beyond case series into widespread practice. The British Heart Foundation (BHF) is sponsoring HOPE-HF, a study of HBP in patients with first degree AV block and LV impairment. A new development, in those patients where HBP cannot correct LBBB is direct stimulation of the LBBB trans-septally, which is now well described with excellent pacing thresholds.14
Conclusion
Cardiac pacing has matured, yet over the last 60 years there have been major innovations, such as dual-chamber pacing, rate response, ICDs and CRT. There are exciting developments encompassing all aspects of cardiac pacing and defibrillation from implantation to follow-up. There is still the potential for developments in the field of biological pacemakers to completely change practice.
Key messages
- Update on current pacing practice
- Future developments for pacemaker clinics, leadless pacing
- Benefits of permanent His-bundle pacing
Conflict of interest
PF has received travel grants from Biotronik and Medtronic; he has also been involved in research trials for Abbott, Biotronik and Medtronic.
Articles in the Pacing supplement
Introduction
A brief history of cardiac pacing in the UK
Cardiac resynchronisation therapy – developments in heart failure management
Drugs with devices in the management of heart failure
His-bundle pacing: UK experience and HOPE for the future!
Leadless pacing
Techniques in pacemaker and defibrillator lead extraction
Remote monitoring
Remote follow-up of ICS: a physiologist’s experience
References
1. Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol 2006;8:17–81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232561/
2. Cantillon DJ, Exner DV, Badie N et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC: Clinical Electrophysiology 2017;3:1296–305. https://doi.org/10.1016/j.jacep.2017.05.007
3. Birnie DH. A randomized controlled trial of continued versus interrupted direct oral anti-coagulant at the time of device surgery – BRUISE CONTROL-2. Presented at the American Heart Association Annual Scientific Sessions (AHA 2017), Anaheim, CA, 12 November 2017.
4. Matsumoto AH, Reifsnyder AC, Hartwell GD et al. Reducing the discomfort of lidocaine administration through pH buffering. J Vasc Interv Radiol 1994;5:171–5. https://doi.org/10.1016/S1051-0443(94)71478-0
5. Van Gelder IC, Healey JS, Crijns H et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. https://doi.org/10.1093/eurheartj/ehx042
6. Nazarian S, Hansford R, Rahsepar AA et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med 2017;377:2555–64.
7. Shun-Shin MJ, Zheng SL, Cole GD et al. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta-analysis of 8567 patients in the 11 trials. Eur Heart J 2017;38:1738–46. https://doi.org/10.1093/eurheartj/ehx028
8. Brignole M, Occhetta E, Bongiorni MG et al. SAFE-ICD Study Investigators. Clinical evaluation of defibrillation testing in an unselected population of 2,120 consecutive patients undergoing first implantable cardioverter-defibrillator implant. J Am Coll Cardiol 2012;60:981–7. https://doi.org/10.1016/j.jacc.2012.05.014
9. Leyva F. Cardiac resynchronization therapy guided by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:64. https://doi.org/10.1186/1532-429X-12-64
10. Behar JM, Bostock J, Li A et al. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol 2015;26:540–6. https://doi.org/10.1111/jce.12625
11. Leyva F, Zegard A, Qiu T et al. Cardiac resynchronization therapy using quadripolar versus non-quadripolar left ventricular leads programmed to biventricular pacing with single-site left ventricular pacing: impact on survival and heart failure hospitalization. J Am Heart Assoc 2017;6:e007026. https://doi.org/10.1161/JAHA.117.007026
12. Mandrola J. His Bundle Pacing Hits the Mainstream at HRS 2017. 15 May 2017. Available at: www.medscape.com/viewarticle/879996
13. Huang W, Su L, Wu S et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–1736.e3.
14. Vijayaraman P, Subzposh FA, Dandamudi G et al. Permanent His bundle pacing reduces mortality/morbidity in pacemaker population compared to right ventricular pacing. Heart Rhythm Scientific Sessions 2017.

