Current landscape
For 60 years, right ventricular pacing has been the standard of care for patients with bradycardia who require cardiac pacing. However, while right ventricular pacing is very effective in preventing bradycardia, it produces non-physiological ventricular activation. The ventricular activation wave-front proceeds slowly via cell-to-cell conduction from the pacing site within the right ventricle, resulting in electrical dyssynchrony with delayed left ventricular activation. This non-physiological activation may lead to left ventricular impairment.1,2 Furthermore, right ventricular pacing, when delivered to patients with ventricular impairment, increases the incidence of heart failure3,4 and death.4
Methods to mitigate the detrimental effects of ventricular pacing have included programming strategies to reduce exposure to right ventricular pacing. These device-based algorithms can successfully reduce right ventricular pacing, but do so at the expense of allowing abnormally long, non-physiological, atrioventricular (AV) delays.5 These algorithms are not useful in pacing dependent patients.
Alternative ventricular pacing sites, such as septal pacing, deliver a narrower QRS duration, but ventricular activation still occurs, predominantly via slow cell-to-cell conduction, and produces non-physiological ventricular activation patterns. This may explain why it has not consistently been found to be superior to right ventricular pacing.6,7
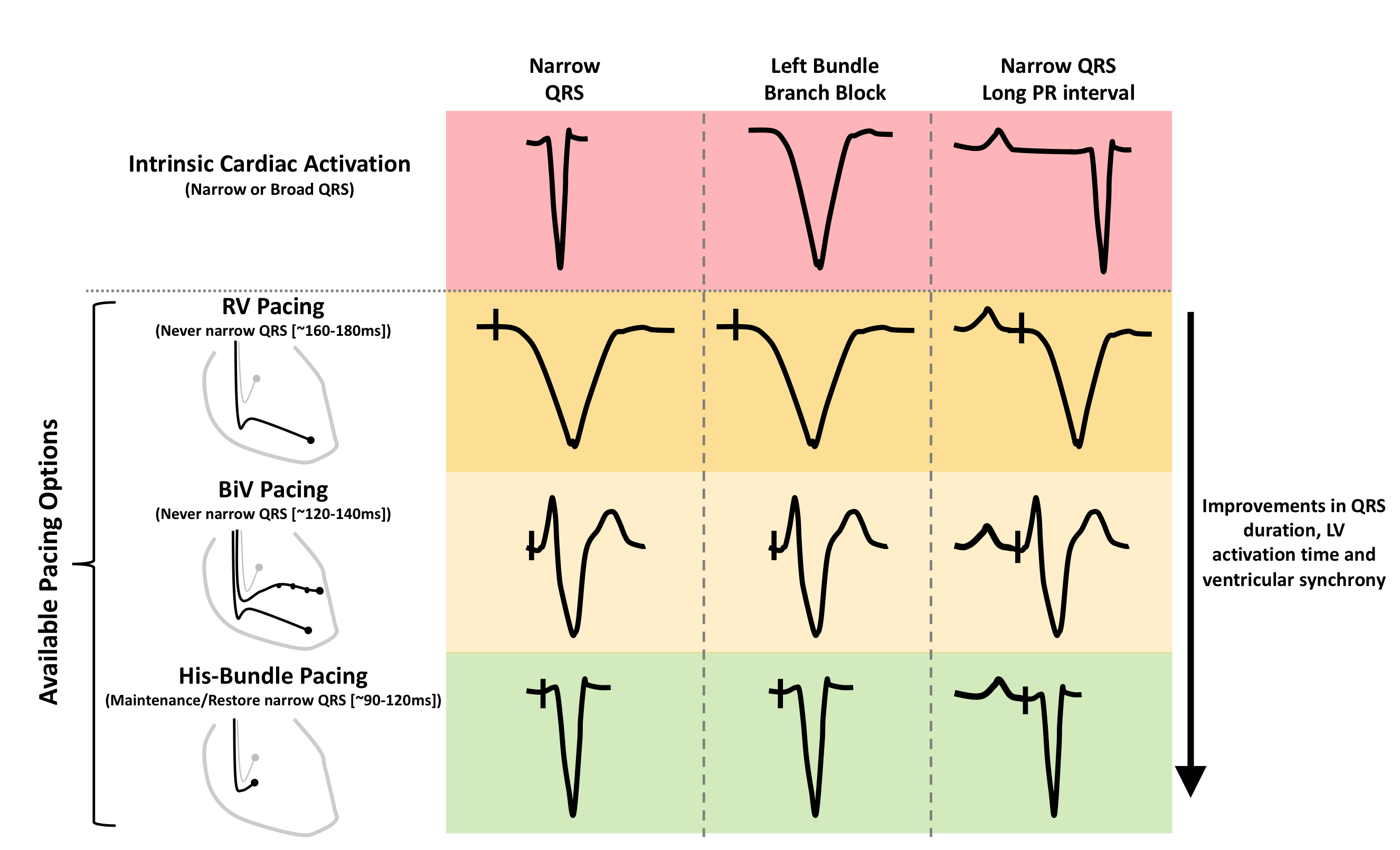
Biventricular pacing delivers more rapid ventricular activation compared with right ventricular apical pacing, however, activation is still significantly longer than during normal physiological activation.8,9 Therefore, when applied to patients with narrow QRS, biventricular pacing causes ventricular dyssynchrony, which may explain the harmful effects when applied to this population (figure 1).10
His-bundle pacing: physiological pacing
The first report of permanent His-bundle pacing was in 2000, this ground-breaking study demonstrated the feasibility of permanent His-bundle pacing.11 In patients with narrow QRS duration, His-bundle pacing allows identical ventricular activation patterns with preservation of QRS duration and morphology (figure 2).

The implant procedure
Dedicated delivery sheaths are now available to facilitate the delivery of the lead to the His bundle. The atrial component of the His bundle, which sits above the tricuspid valve on the right atrial–left ventricular part of the membranous septum, is targeted. The 3830 Select Secure lead (Medtronic Minneapolis, MN) is currently the lead of choice for His pacing, the screw is the active electrode and facilitates direct stimulation of the His-bundle fibres.
Unlike right ventricular pacing, in which only fluoroscopy is traditionally used to position the lead, His-bundle pacing ideally requires identification of a specific electrical signal. In order to locate the His bundle, the electrical signal from the lead tip is displayed in real-time and used to guide the position of the lead. The lead should ideally be deployed in a position where there is a clear His signal, and the local ventricular electrocardiogram (ECG) is ideally at least twice the amplitude of the preceding atrial signal (figure 3). Implant success rates are currently in the region of >80–92%.12-16

When to consider His-bundle pacing?
Large randomised-controlled trials showing a clear clinical benefit of His pacing are not yet available. However, data from observational studies show promising results with respect to feasibility and safety.12-16
The HOPE-HF (His Optimised Pacing Evaluated for Heart Failure) study is the largest double-blinded, randomised-controlled trial of His pacing, which is currently recruiting patients with a long PR interval and heart failure, to assess whether AV delay optimisation delivered using His-bundle pacing improves exercise capacity (Clinical Trials NCT02671903).17
His pacing for bradycardia
His-bundle pacing to facilitate AV node ablation
The first report of permanent His-bundle pacing was in a group of 18 patients who received AV node ablation to allow rate control of atrial fibrillation.11 All patients had pre-existing impairment of left ventricular function. Despite not having dedicated implant tools, permanent His-bundle pacing could be achieved in 66% of patients. Pacing thresholds remained stable over the follow-up period and, encouragingly, left ventricular function improved even with chronic ventricular pacing activation. Feasibility has subsequently been confirmed in larger observational studies.18,19
His-bundle pacing to treat bradycardia due to conduction system disease
The observation that His-bundle pacing is feasible following AV node ablation was an important step forward. However, there were initial concerns about whether His pacing would be reliable in patients with more distal conduction system disease. Encouragingly, a number of observational studies have demonstrated that His pacing is feasible in this population of patients, including patients with permanent complete heart block.12,15,16
In a recently published long-term observational study of patients with a bradycardia indication for pacing, 304 patients (92% of those attempted) received His pacing and were compared to 433 patients who received right ventricular pacing. During mean follow-up of 725 ± 423 days, His pacing was found to be reliable. It was noted that pacing thresholds were higher in the His pacing group, 1.30 ± 0.85 V at 0.79 ± 0.26 ms versus 0.59 ± 0.42 V at 0.5 ± 0.03 ms (p<0.01), and the sensed R-wave was also lower in the His-pacing group, but this did not appear to result in significant clinical consequences.
This observational comparison showed that the His-pacing group experienced significantly lower death, heart-failure hospitalisations or a need to upgrade to biventricular pacing compared to the right ventricular pacing group (25% vs. 31.6%; p=0.02), this difference was most pronounced in patients who had a higher burden (>20%) of ventricular pacing (25.3% vs. 35.6%; p=0.02).16
Adequately powered randomised-controlled trials are now required to establish whether this promising result translates into improved outcomes when tested in a rigorous and bias-resistant fashion. Positive trials would revolutionise the way anti-bradycardia pacing is delivered.
His cardiac resynchronisation therapy in patients with bundle branch block
Biventricular pacing is proven to reduce morbidity and mortality when delivered to patients with impaired left ventricular systolic function and left-bundle branch block.20-22 However, despite treatment, symptom burden and mortality rates remain high.20-22
Biventricular pacing delivers imperfect ventricular resynchronisation, with only modest reductions in QRS duration and ventricular activation time in patients with left-bundle branch block.8 More effective ventricular resynchronisation has the potential to deliver additional improvements in cardiac function, over and above those seen with current biventricular pacing.9
His-bundle pacing shows promise as an alternative method for delivering ventricular resynchronisation therapy. In patients with bundle branch block, His pacing may overcome the branch block and result in normalisation of ventricular activation time and pattern.23,24
There are several theories for how His-bundle pacing can overcome bundle branch block. The simplest is that bundle branch block often occurs as a result of fasicular conduction block or delay within the His bundle. Which can be overcome by positioning the pacing lead distal to the site of block and thus permitting electrical bypass and reversal of the block (figure 4).

Dramatic improvements in QRS duration have been observed in 70–90% of patients with bundle branch block.13,14,25 In a recently published study, QRS duration was reduced from 157 ± 33 ms to 117 ± 18 ms.13 In these observational studies, improvements in left ventricular ejection fraction and symptoms were observed.
In the study by Sharma et al., His pacing was attempted as an alternative to biventricular pacing, either when the operator failed to insert a left ventricular lead, if the patient was felt to be a non-responder to biventricular pacing or in patients with AV block, bundle branch block, or with an existing high ventricular pacing burden. During mean follow-up of 14 months, significant narrowing of QRS occurred and this was associated with an increase in left ventricular ejection fraction from 30% ± 10% to 43% ± 13% (p=0.0001), and improvement in New York Heart Association (NYHA) functional class from 2.8 ± 0.5 to 1.8 ± 0.6 (p=0.0001) with His pacing.13
In another study, patients with class 1 indications for cardiac resynchronisation therapy (CRT) received a His lead rather than a biventricular pacing system. Of the 21 patients in whom implantation was attempted, His-bundle pacing was successfully implanted in 16. A significant reduction in mean QRS was observed, with narrowing from 180 ± 23 ms to 129 ± 13 ms (p<0.0001). During the follow-up period, median NYHA functional class improved from III to II (p<0.001), and mean left ventricular ejection fraction and internal dimensions in diastole improved from 27% ± 10% to 41% ± 13% (p<0.001), and from 5.4 ± 0.4 cm to 4.5 ± 0.3 cm (p<0.001).14
These findings provide support for the notion that His-resynchronisation therapy may be an alternative method for delivering ventricular resynchronisation.
His pacing has the advantage that contrast is not required during the procedure, phrenic nerve stimulation does not occur, and implantation is not limited by the constraints of the coronary sinus anatomy.
Prolonged PR interval, a new target for pacing therapy for heart failure?
PR prolongation is associated with adverse outcomes, this has been observed both in observational studies in people with ischemic heart disease,26 and patients with heart failure.27,28 In the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial, non-left bundle branch block (LBBB) patients in the control group had a threefold higher risk of death or heart failure if their PR interval was prolonged.27
Acute studies have shown that improving left ventricular filling by shortening AV delay improves acute cardiac function, as reflected by changes in left ventricular dP/dtmax, stroke volume, coronary artery flow and systolic blood pressure.29-32 Indeed, much of the beneficial effect of biventricular pacing appears to be delivered through improvements in ventricular filling occurring as a result of shortening AV delay.9
A prolonged PR interval may, therefore, represent an alternative target for pacing therapy for heart failure. Shortening prolonged PR intervals with pacing is a well-established concept, however, when delivered to patients with a normal QRS duration both right ventricular and biventricular pacing result in non-physiological ventricular activation and ventricular dyssynchrony. This is likely to offset some of the beneficial effects obtained through shortening of AV delay optimisation (figure 1).
His pacing, allows AV delay to be shortened without introducing ventricular dyssynchrony.33 AV delay optimisation delivered with His-bundle pacing to patients with a prolonged PR interval, a normal QRS or right-bundle branch block (RBBB) and heart failure resulted in acute haemodynamic improvements.34 The observed effect size was around 60% of that seen when biventricular pacing is delivered to patients with LBBB.
We are investigating the longer-term effects of AV-optimised His pacing in this population of patients in the HOPE-HF trial.17 This is a UK multi-centre, double-blind, randomised, cross-over study, which is evaluating the long-term effects of AV-optimised His pacing. We aim to recruit 160 patients with PR prolongation, left ventricular impairment, and either narrow QRS (≤140 ms) or RBBB. All patients receive a CRT-pacemaker or CRT-defibrillator device with a His lead and are randomised to have six months AV-optimised His pacing and six months with back-up pacing only. The primary end point of the trial is exercise capacity measured using cardiopulmonary exercise testing.
If the trial produces positive results, then this would provide support for extending pacing therapy for heart failure to a group of patients who are currently not eligible for treatment with biventricular pacing.
Conclusion
Permanent His-bundle pacing allows ventricular stimulation to be delivered via the natural cardiac conduction system. When used for bradycardia pacing, it has the advantage that it may avoid the harmful consequences of pacing-induced ventricular dyssynchrony.
His pacing shows promise as an alternative method for delivering CRT.
AV-optimised His pacing improves acute cardiac function in patients with heart failure, and a prolonged PR interval. The randomised HOPE-HF trial is currently investigating whether these acute benefits translate into longer-term improvements.
Permanent His-bundle pacing has the potential to change the face of cardiac pacing, but adequately powered randomised trials are awaited to confirm the promising results from the early work.
Key messages
- Permanent His-bundle pacing is a safe and feasible approach for providing cardiac activation
- Permanent His-bundle pacing provides physiological ventricular activation
- Observational studies support the use of His-bundle pacing in both bradycardia and ventricular resynchronisation settings
- Randomised-controlled trials are needed to confirm the theoretical benefits
Conflict of interest
None declared.
Articles in this supplement:
Introduction
Current and future perspectives on cardiac pacing
A brief history of cardiac pacing in the UK
Cardiac resynchronisation therapy – developments in heart failure management
Drugs with devices in the management of heart failure
Leadless pacing
Techniques in pacemaker and defibrillator lead extraction
Remote monitoring
Remote follow-up of ICS: a physiologist’s experience
References
1. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol 2009;54:764–76. https://doi.org/10.1016/j.jacc.2009.06.006
2. Tse H-F, Lau C-P. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol 1997;29:744–9. https://doi.org/10.1016/S0735-1097(96)00586-4
3. Sweeney MO, Hellkamp AS, Ellenbogen KA et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. https://doi.org/10.1161/01.CIR.0000072769.17295.B1
4. The DAVID Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator. JAMA 2002;288:3115. https://doi.org/10.1001/jama.288.24.3115
5. Shurrab M, Healey JS, Haj-Yahia S et al. Reduction in unnecessary ventricular pacing fails to affect hard clinical outcomes in patients with preserved left ventricular function: a meta-analysis. Europace 2017;19:282–8. https://doi.org/10.1093/europace/euw221
6. Kypta A, Steinwender C, Kammler J, Leisch F, Hofmann R. Long-term outcomes in patients with atrioventricular block undergoing septal ventricular lead implantation compared with standard apical pacing. Europace 2008;10:574–9. https://doi.org/10.1093/europace/eun085
7. Kaye GC, Linker NJ, Marwick TH et al. Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the Protect-Pace study. Eur Heart J 2015;36:856–62. https://doi.org/10.1093/eurheartj/ehu304
8. Ploux S, Eschalier R, Whinnett ZI et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm 2015;12:782–91. https://doi.org/10.1016/j.hrthm.2014.12.031
9. Jones S, Lumens J, Sohaib SMA et al. Cardiac resynchronization therapy: mechanisms of action and scope for further improvement in cardiac function. Europace 2017;19:1178–86. https://doi.org/10.1093/europace/euw136
10. Ruschitzka F, Abraham WT, Singh JP et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405. https://doi.org/10.1056/NEJMoa1306687
11. Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000;101:869–77. https://doi.org/10.1161/01.CIR.101.8.869
12. Vijayaraman P, Naperkowski A, Subzposh FA et al. Permanent His-bundle pacing: long-term lead performance and clinical outcomes. Heart Rhythm 2018;15:696–702. https://doi.org/10.1016/j.hrthm.2017.12.022
13. Sharma PS, Dandamudi G, Herweg B et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm 2018;15:413–20. https://doi.org/10.1016/j.hrthm.2017.10.014
14. Ajijola OA, Upadhyay GA, Macias C, Shivkumar K, Tung R. Permanent His-bundle pacing for cardiac resynchronization therapy: initial feasibility study in lieu of left ventricular lead. Heart Rhythm 2017;14:1353–61. https://doi.org/10.1016/j.hrthm.2017.04.003
15. Sharma PS, Dandamudi G, Naperkowski A et al. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm 2015;12:305–12. https://doi.org/10.1016/j.hrthm.2014.10.021
16. Abdelrahman M, Subzposh FA, Beer D et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol 2018;71:2319–30. https://doi.org/10.1016/j.jacc.2018.02.048
17. Keene D, Arnold A, Shun-Shin MJ et al. Rationale and design of the randomized multicentre His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) trial. ESC Heart Fail 2018 (published online July 9th 2018).
18. Huang W, Su L, Wu S et al. Benefits of permanent His bundle pacing combined with atrioventricular node ablation in atrial fibrillation patients with heart failure with both preserved and reduced left ventricular ejection fraction. J Am Heart Assoc 2017;6:e005309. https://doi.org/10.1161/JAHA.116.005309
19. Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace 2017;19(suppl 4):iv10–iv16. https://doi.org/10.1093/europace/eux263
20. Cleland JGF, Daubert J-C, Erdmann E et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. https://doi.org/10.1056/NEJMoa050496
21. Cleland JG, Abraham WT, Linde C et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–56. https://doi.org/10.1093/eurheartj/eht290
22. Moss AJ, Hall WJ, Cannom DS et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. https://doi.org/10.1056/NEJMoa0906431
23. Teng AE, Massoud L, Ajijola OA. Physiological mechanisms of QRS narrowing in bundle branch block patients undergoing permanent His bundle pacing. J Electrocardiol 2016;49:644–8. https://doi.org/10.1016/j.jelectrocard.2016.07.013
24. Teng AE, Lustgarten DL, Vijayaraman P et al. Usefulness of his bundle pacing to achieve electrical resynchronization in patients with complete left bundle branch block and the relation between native QRS axis, duration, and normalization. Am J Cardiol 2016;118:527–34. https://doi.org/10.1016/j.amjcard.2016.05.049
25. Lustgarten DL, Crespo EM, Arkhipova-Jenkins I et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: a crossover design comparison. Heart Rhythm 2015;12:1548–57. https://doi.org/10.1016/j.hrthm.2015.03.048
26. Crisel RK, Farzaneh-Far R, Na B, Whooley MA. First-degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: data from the Heart and Soul Study. Eur Heart J 2011;32:1875–80. https://doi.org/10.1093/eurheartj/ehr139
27. Stockburger M, Moss AJ, Klein HU et al. Sustained clinical benefit of cardiac resynchronization therapy in non-LBBB patients with prolonged PR-interval: MADIT-CRT long-term follow-up. Clin Res Cardiol 2016;105:944–52. https://doi.org/10.1007/s00392-016-1003-z
28. Salden FCWM, Kutyifa V, Stockburger M, Prinzen FW, Vernooy K. Atrioventricular dromotropathy: evidence for a distinctive entity in heart failure with prolonged PR interval? Europace 2018;20:1067–77. https://doi.org/10.1093/europace/eux207
29. Nishimura RA, Hayes DL, Holmes DR, Tajik J. Mechanism of hemodynamic improvement by dual-chamber pacing for severe left ventricular dysfunction: an acute Doppler and catheterization hemodynamic study. J Am Coll Cardiol 1995;25:281–8. https://doi.org/10.1016/0735-1097(94)00419-Q
30. Kyriacou A, Whinnett ZI, Sen S et al. Improvement in coronary blood flow velocity with acute biventricular pacing is predominantly due to an increase in a diastolic backward-travelling decompression (suction) wave. Circulation 2012;126:1334–44. https://doi.org/10.1161/CIRCULATIONAHA.111.075606
31. Whinnett ZI, Francis DP, Denis A et al. Comparison of different invasive hemodynamic methods for AV delay optimization in patients with cardiac resynchronization therapy: implications for clinical trial design and clinical practice. Int J Cardiol 2013;168:2228–37. https://doi.org/10.1016/j.ijcard.2013.01.216
32. Whinnett ZI, Davies JER, Willson K et al. Haemodynamic effects of changes in atrioventricular and interventricular delay in cardiac resynchronisation therapy show a consistent pattern: analysis of shape, magnitude and relative importance of atrioventricular and interventricular delay. Heart 2006;92:1628–34. https://doi.org/10.1136/hrt.2005.080721
33. Arnold A, Shun-Shin M, Keene D et al. Shortening AV delay appears to be the major mechanism through which cardiac resynchronization therapy improves acute cardiac function. Heart Rhythm 2017;14:C-PO02-172.
34. Sohaib SMA, Wright I, Lim E et al. Atrioventricular optimized direct His bundle pacing improves acute hemodynamic function in patients with heart failure and PR interval prolongation without left bundle branch block. JACC: Clin Electrophysiol 2015;1:582–91. https://doi.org/10.1016/j.jacep.2015.08.008

