This year’s fascinating and thought-provoking 13th Annual Scientific Meeting of the Cardiorenal Forum brought together experts from the closely linked specialties of nephrology, cardiology and diabetes. Held in London on 5th October 2018, the meeting addressed a multidisciplinary audience and covered a variety of subjects, from recently updated guidelines to recently published trials and intriguing case-based discussion. Dr Panagiota Mitropoulou reports on its highlights.
Which diabetes drug to choose for which patient?
The day’s first keynote session was given by Dr David Strain (University of Exeter, Royal Devon and Exeter Hospital). He discussed different classes of diabetes medications and their advantages and disadvantages, emphasising the importance of early initiation and early intensification of treatment. He also highlighted the serious problem of non-adherence to treatment, as two thirds of patients with diabetes do not take their medication as prescribed. He insisted that an important part of our job as clinicians is to address whether the tablets we recommend will be taken.
Whilst the first-line treatment for type 2 diabetes is metformin, guidelines are not clear as to what the next step should be. Dr Strain explained that sulphonylureas are falling out of favour, especially since the combination of metformin with sulphonylureas was shown to have increased mortality in the UKPDS (UK Prospective Diabetes Study). Thiazolidinediones can be considered but Dr Strain advocated using one of the new generation drugs, DPP-4 (dipeptidyl peptidase 4) inhibitors, SGLT2 (sodium-glucose transport protein 2) inhibitors and GLP-1 (glucagon-like peptide 1) agonists. The decision should be tailored to the patient’s needs and comorbidities, he said.
DPP-4 inhibitors (gliptins) are beneficial in early diabetes, can be used in renal failure and have good safety data in older patients. SGLT2 inhibitors (gliflozins) can be used safely in heart failure and have demonstrated benefit with respect to improving cardiovascular outcomes (seen early). They require preserved renal function (estimated glomerular filtration rate [eGFR] >60), however, and their side-effect profile may limit their use in the elderly. GLP-1 agonists (of which glutides seem to be better) are of particular benefit to those with atherosclerotic disease but being injectable may lead to some patients not wanting to use these medications.
Is a 130/80 target the new 140/90?
Hypertension was in the spotlight during the meeting and addressed by two speakers: Professors Anthony Heagerty (University of Manchester) and Una Martin (University of Birmingham). With recently published European Society of Hypertension (ESH),1 American Hypertension Association (AHA)2 and Hypertension Canada (CHEP)3 guidelines, there was much to cover.
Professor Martin presented these guidelines and compared their differences. Regarding diagnosis, it is recommended that out of office measurements with either ambulatory blood pressure (BP) monitoring or home measurements are essential. The classification of hypertension remains the same as in previous guidelines (table 1). There is some discrepancy amongst the three guidelines regarding when to start treatment: the European guidelines putt the emphasis on lifestyle modification for grade 1 hypertension with the exception of high-risk patients, while the American guidelines advise treating all patients with BP >140/90 mmHg and to lower this threshold further to >130/80 mmHg for high-risk patients.
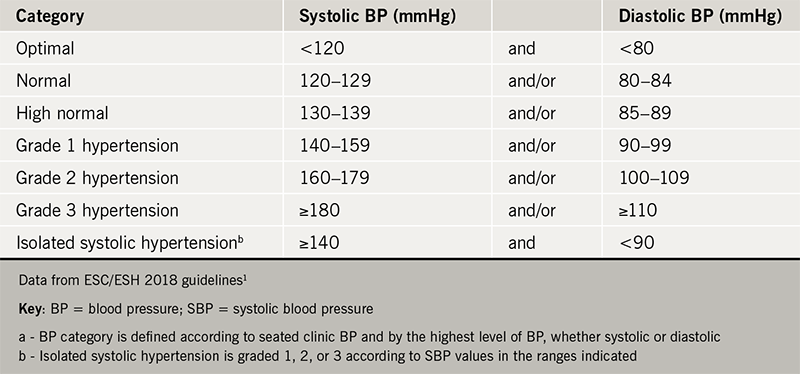
Another key point is that the control of hypertensive patients should be tighter than previously. Advice is now for a target of <130/80 mmHg in younger patients (age <65 years) and <140/80 mmHg in patients older than 65 (irrespective of risk factors, provided that the treatment is tolerated). Interestingly, the risk of falls has not be shown to be increased with tight BP control in any age group, although other adverse effects (hypotension, orthostatic hypotension, acute kidney injury and electrolyte abnormalities) have been found to be more common.
Another major change compared to previous recommendations is that treatment should be initiated with two agents rather than one (with the exception of elderly and low-risk patients). It is recognised that adherence will be a significant hindrance thus recommendations advise a single (combination) tablet should be chosen when possible. Interestingly, the new drug-treatment strategy for hypertension does not account for ethnicity – It recommends that treatment should be initiated with an angiotensin-converting-enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) plus a calcium channel blocker (CCB) or thiazide/thiazide-type diuretic, with the next step being a triple combination of these medications. The addition of spironolactone/other diuretic/alpha-blocker/beta blocker is reserved for resistant hypertension. More specifically, initial therapy for patients with heart failure with reduced ejection fraction (HFrEF) should include ACE inhibitor/ARB plus beta blocker plus diuretic (thiazide or loop diuretic), with the addition of spironolactone for resistant cases.
Looking forward, it is interesting to see how these guidelines will be adopted in clinical practice. Publication of the updated National Institute for Health and Care Excellence guidelines (NICE), expected in March 2019, may help to crystallise our practice.
Advances in percutaneous treatment of valvular disease
As our patient population grows older, aortic valve disease becomes increasingly common. Trans-catheter valve therapy has changed the landscape of the management of valve disease. Professor Phil MacCarthy (King’s College, London) took us on a tour through the current landscape of transcatheter valve therapy and discussed pioneering techniques for the percutaneous treatment of valvular disease.
He started by highlighting the difficulties clinicians face in defining the point at which patients become symptomatic and therefore fulfil the criteria for valve surgery, as many patients have several other comorbidities, which could account for their symptoms. He then went on to address the complex relationship between renal failure and aortic valve interventions. Indeed, renal failure accelerates aortic valve calcification and stenosis, while renal failure significantly increases the peri-operative risk of surgical aortic valve replacement or transcatheter aortic valve implantation (TAVI) and influences the prognosis, lessening the prognostic gain of these procedures. On the other hand, the procedures can worsen renal failure. In this context, he addressed the techniques that can be used to protect the kidneys during TAVI, such as the RenalGuard system, which was validated by the results of the PROTECT TAVI (Prospective Randomized Outcome Study in TAVI Patients Undergoing Periprocedural Embolic Cerebral Protection With the Claret Sentinel™ Device) trial.
Dilemmas in selecting the right population for TAVI were discussed. TAVI has been shown to be beneficial for patients who have been turned down for cardiac surgery. It is of note that the first TAVI trial published within this patient population demonstrated an impressive number needed to treat (NNT) of four to save a life at 12 months.4 TAVI also seems to be the preferred treatment in the high-risk surgical patient population compared to high-risk surgery. The intermediate-risk patient group is slightly more controversial – TAVI seems to be at least as good as cardiac surgery, as demonstrated by trials such as PARTNER II and CoreValve.
The above resonates with the fact that TAVI is an increasingly simplified procedure, carried out with local anaesthetic and a femoral approach for the vast majority of patients. Professor MacCarthy explained that certain patient populations, such as renal patients with significant peripheral arterial disease, may require alternative approaches e.g. a transapical or transcaval (via the inferior vena cava) approach. Patients are encouraged to mobilise early after TAVI and are usually discharged on day two postoperatively.
One of the most important complications of TAVI is paravalvular leak, while 10–20% of patients require permanent pacemaker insertion.
TAVI has had significant implications on how we manage surgical valve replacement and the choice between metallic and bioprosthetic aortic valve implantation. More specifically, we now have the option of performing TAVI on a failing bioprosthetic aortic valve. This may influence the cardiac surgeons’ and patients’ decision on whether to implant a metallic or tissue aortic valve. It is likely this has contributed to a decrease in the number of mechanical aortic valves.
Moving on to focus on the mitral valve, Professor MacCarthy explained that the most common percutaneous intervention performed on the mitral valve is with the MitraClip. The recently published results of COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) study suggest that MitraClip may be beneficial for functional mitral regurgitation in selective patients with severe heart failure when compared to optimal medical treatment.5 This is in contrast to another recent publication suggesting no benefit. More data are required to define the impact of these findings on guidelines and clinical practice.
RAAS inhibitors – nephrotoxins? Mind your language!
In this enlightening talk, Dr Charles Tomson (Freeman Hospital, Newcastle upon Tyne) demystified the management of deteriorating renal function in patients with heart failure who are on medications that block the renin-angiotensin-aldosterone system (RAAS).
It is well recognised that RAAS inhibitors benefit patients with HFrEF. It is also well known that RAAS inhibitors slow down the progression of chronic kidney disease and proteinuria, an effect explained by the hyperfiltration theory. Due to their vasodilating action on the efferent arterioles of the kidney, however, RAAS inhibitors often reduce the eGFR, particularly in patients with pre-existing heart failure and kidney disease.
The main difficulty lies in the fact that there is not enough evidence to guide our decisions in this situation. The threshold for what constitutes an ‘acceptable’ rise in creatinine varies in different guidelines (30% according to NICE guidance or 50% according to the European Society of Cardiology guidelines). It is therefore very challenging for the physician to decide whether to continue or stop RAAS inhibitors in this patient group. Dr Tomson explained that many general physicians tend to either stop these medications too early or leave patients off them for too long, depriving them from the benefits of the RAAS blockade. This is partly due to the widespread description of RAAS inhibitors as ‘nephrotoxins’, which gives the wrong impression that these medications have a detrimental effect on the kidneys. On the contrary, there is evidence that a short-term fall in eGFR after initiation of RAAS is associated with long-term improvement in prognosis in patients with chronic kidney disease.
One of the important messages that Dr Tomson stressed is that we often fail to follow up the renal function of patients, two months after patients have been started on RAAS inhibitors, as guidelines strongly advise.
Based on the physiological effect of RAAS inhibitors increasing the renal tubular blood flow (which is irrespective of their action on the efferent arterioles and the eGFR), Dr Tomson suggested that continuing these medications during acute illness may be beneficial for the kidney, contrary to common belief that they should be stopped (‘sick day rule’). He clarified this by explaining that the renal tubules are the most vulnerable part of the kidney during acute kidney injury (AKI), and therefore increasing their blood flow may improve the outcome after AKI. However, for the time being we only have observational data to support this theory, which needs to be tested with randomised controlled trials.
Dr Tomson finished his talk by summarising the Renal Association (RA) and British Society for Heart Failure (BSH) guidelines, which he hoped would provide a sound basis on which physicians should base decisions. These recommendations include:
- making careful clinical assessment of fluid status
- using the dose of diuretics needed to achieve decongestion
- focusing less on biochemistry results.
Moreover, it is advised that we should make every effort to keep HFrEF patients on RAAS inhibitors. In this context, decreasing the dose and continuing the medication should be considered for HFrEF patients with a rise in creatinine between 30–50%. If creatinine rises by more than 50%, RAAS inhibitors should be temporarily discontinued with every effort made to restart treatment once eGFR improves. Finally, for patients with severe renal dysfunction (eGFR <20), a decision on whether to stop RAAS inhibitors should be based on the presence or not of symptomatic uraemia rather than baseline kidney function.
Finally, looking at the management of hyperkalaemia in this patient population, the threshold for stopping RAAS inhibitors should be a serum potassium of 6 mmol/L (unless the patient is clinically unwell with sepsis, hyperkalaemia and/or AKI, in which case we should consider stopping these medications if potassium >5.5 mmol/L). This is particularly important in the case of patients with decompensated heart failure, with or without AKI, who should be kept on RAAS inhibitors if possible, with the dose of diuretics (loop diuretics +/- thiazide) increased to reduce serum potassium. If RAAS blockers are stopped in patients with HFrEF, then these drugs should be restarted and re-up titrated in due course.
Sudden cardiac death in end-stage renal disease
Professor Philip Kalra (University of Manchester, Salford Royal NHS Foundation Trust) drew our attention to the causes of death in patients with end-stage renal disease on dialysis. It is well recognised that these patients have significantly increased mortality and the common causes of death are different compared to the general population. Indeed, their mortality is 14% per year, while 27% of all-cause mortality is due to sudden cardiac death (SCD) i.e. death within less than an hour of symptom onset in a person who is not known to have a potentially fatal condition. All-cause death rates are higher around the time of the first dialysis session of the week, in patients on typically three dialysis sessions per week. A multitude of factors account for this: hypo- or hyperkalaemia, rapid shift of potassium during dialysis, or quick fluid removal. Other causes consider are the increased prevalence of heart failure, vascular calcification and myocardial fibrosis in dialysis patients.6,7
Until recently, the underlying rhythm causing SCD in this patient population was unknown. Since 2015, five studies including patients from six different countries have been published, all of which point to bradyarrhythmia rather than tachyarrhythmia as being the pre-eminent pattern of serious arrhythmias. Professor Kalra presented the results of some of these studies.
In the CRASH-ILR study,8 the only UK-based trial, 30 haemodialysis patients received an implantable loop recorder (ILR) and ECG data from the loop recorders were transmitted at each haemodialysis session using a remote monitoring system. The study reported:
- eight deaths – two SCDs and six due to generalised deterioration/sepsis
- five (20%) patients had a primary outcome event – two died of SCD and three required pacemaker implantations for bradyarrhythmias, all of which were asymptomatic. Of the patients who died of SCD, one was found to have died of ventricular fibrillation after interrogation of the ILR, while the other had had their device explanted before their death due to infection and therefore no data were available at the time of death.
In a similar study9 of 50 Australian haemodialysis patients with a left ventricular ejection fraction > 35% who were monitored with ILRs, bradycardia accounted for 65% of all significant arrhythmias detected. Of the eight SCDs, six had captured rhythm data that showed either bradycardia or asystole. Only 0.8% of all arrhythmic events were related to ventricular tachycardia or ventricular fibrillation.
A consistent message amongst these ILR studies is that although incidental ventricular tachyarrhythmia is common, fatal rhythm disturbances may well be bradycardic. Further analysis is required to see if expanded use of ILRs in the haemodialysis population might help risk stratify those at risk of life-threatening arrhythmias, and whether detection and intervention towards asymptomatic bradyarrhythmias might translate into a life prolonging intervention.
Interactive cases
The interactive clinical cases are always a popular session at the meeting. Podcasts of these are available to view on: www.bjcardio.co.uk/podcasts. Watch Dr Charles Ferro (University Hospitals Birmingham) talk about anticoagulation and chronic kidney disease; Dr Paul Foley (Great Western Hospital, Swindon) talk about iron deficiency, and Professor Neil Sheerin (Newcastle University) talk about atypical haemolytic uraemic syndrome
Acknowledgements
The Cardiorenal Forum would like to thank the following companies for their generous support of the 2018 meeting: Alexion, Amgen, Amicus, Bayer, Bristol Myers Squibb/Pfizer, Menarini, Novartis, Novo Nordisk, Pharmacosmos, Sanofi, Vifor Pharma and Vifor Fresenius.
It also thanks the British Society for Heart Failure, The Renal Association and The Irish Nephrology Society for their endorsement of the 2018 meeting.
Save the date
Next year’s 14th Annual Scientific Meeting of the Cardiorenal Forum will take place Friday 4th October 2019 at the King’s Fund Centre, London. Contact [email protected] for more information or look at the website www.cardiorenalforum.com, which will also carry full details of the next meeting as they become available.
Panagiota Mitropoulou
Cardiology Specialty Registrar
Wessex Deanery
References
1. Williams B et al. ESC/ESH Guidelines for the management of arterial hypertension, Eur Heart J 2018;29:3021–104, https://doi.org/10.1093/eurheartj/ehy339
2. Whelton MB et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Available at: https://www.ahajournals.org/doi/pdf/10.1161/HYP.0000000000000065
3. Leung A et al. (2017), Hypertension Canada’s 2017 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults, Can J Cardiol 2017;33:557–76.
4. Leon MB et al. (2010), Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New Engl J Med 2010;363:1597–607. https://doi.org/10.1056/NEJMoa1008232
5. Stone GW et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018 (Epub ahead of print 23rd September 2018) https://doi.org/10.1056/NEJMoa1806640
6. Kalra PA, Green D, Poulikakos D. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 2018;93:781–3. https://doi.org/10.1016/j.kint.2017.12.005
7. Prabir RC et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle, Kidney Int 2017;93:941–51. https://doi.org/10.1016/j.kint.2017.11.019
8. Roberts PR, Zachariah D, Morgan JM et al. Monitoring of arrhythmia and sudden death in a haemodialysis population: The CRASH-ILR study. PLoS One 2017;12(12) https://doi.org/10.1371/journal.pone.0188713
9. Wong MC, Kalman JM, Pedagogos E et al. Temporal distribution of arrhythmic events in chronic kidney disease: Highest incidence in the long interdialytic period. Heart Rhythm 2015;12:2047–55



