Takotsubo cardiomyopathy (TTC) is characterised by transient left ventricular dysfunction accompanied by apical ballooning of the ventricle. Takotsubo pathophysiology is poorly understood and is often triggered by an emotional or physical stressor. This is a case of a 71-year-old woman who presented with sudden-onset exertional chest pain leading to inferior ST-elevation on electrocardiography (ECG) with a significant troponin rise. Immediate coronary angiography revealed a severe mid-posterior left ventricular (PLV) branch of the right coronary artery stenosis. The left coronary system was normal. Left ventriculogram revealed mid-to-apical ballooning typical of TTC. Considering the disconnect between the coronary and ventriculogram findings, a decision was made to delay percutaneous coronary intervention (PCI). The patient was treated with heart failure medications and symptoms improved. Repeat angiogram of the mid-PLV after a short period of medical therapy revealed no coronary lesion and the left ventricular function had normalised. These findings suggest that coronary artery vasospasm may have contributed to the aetiology in this case of TTC. This case adds weight to previous theories of an interplay between TTC and coronary vasospasm.
Introduction
Takotsubo cardiomyopathy (TTC) is characterised by a transient left ventricular dysfunction, which is classically accompanied by left ventricular apical ballooning and akinesis.1,2 The condition predominantly affects post-menopausal women and involves a neuro-cardiac action often triggered by an emotional or physical stressor.2 While the pathophysiology is not completely understood, postulated mechanisms include catecholamine excess,3 and microvascular dysfunction.4
Case
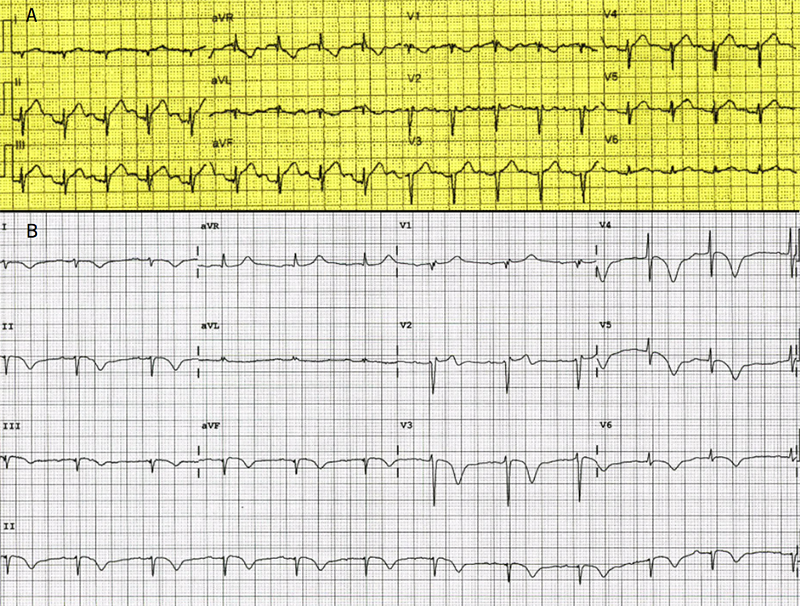
A previously well 71-year-old woman was admitted to hospital via ambulance with sudden-onset angina radiating to the left shoulder and jaw, along with dyspnoea. This was precipitated by exercise on a new treadmill. The patient denied any psychological or emotional stressors. The patient had a history of hypertension, mitral valve prolapse with mild mitral regurgitation and microcystic adenexal carcinoma (in remission). The initial ambulance 12-lead electrocardiogram (ECG) was performed within 30 minutes of symptoms. This showed sinus rhythm with ST-elevation in the inferior leads II, III and aVF (figure 1a), despite lead reversal. A pre-hospital cardiac catheter laboratory activation was instituted. ECG performed in the emergency department en-route to the catheter lab revealed sinus rhythm with diffuse T-wave inversion in multiple leads, QT prolongation of 500 ms and minor ST-elevation in V1 and V2 (figure 1b). Initial bloods revealed a high-sensitivity troponin rise of 986 ng/L (reference range normal <15 ng/L). Transthoracic echocardiography prior to catheter lab transfer revealed apical-mid to apical-left ventricular akinesis, the estimated left ventricular ejection fraction was 44 ± 5%.
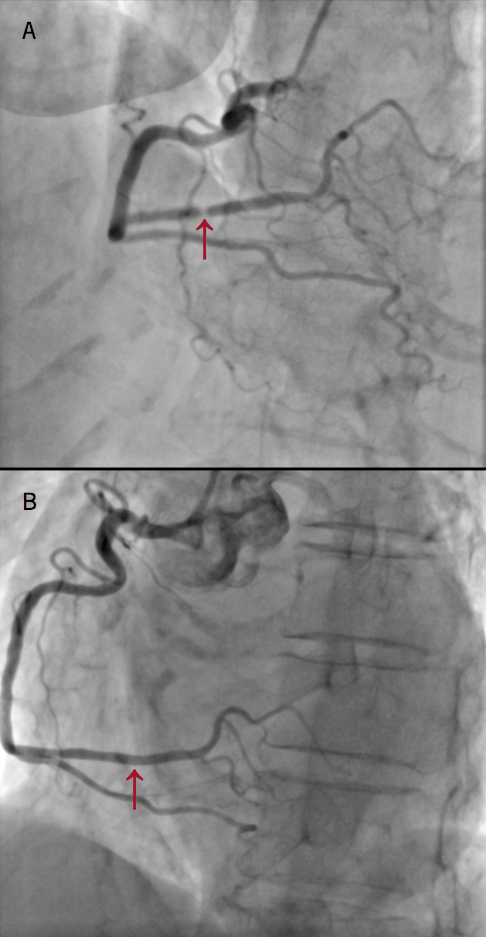
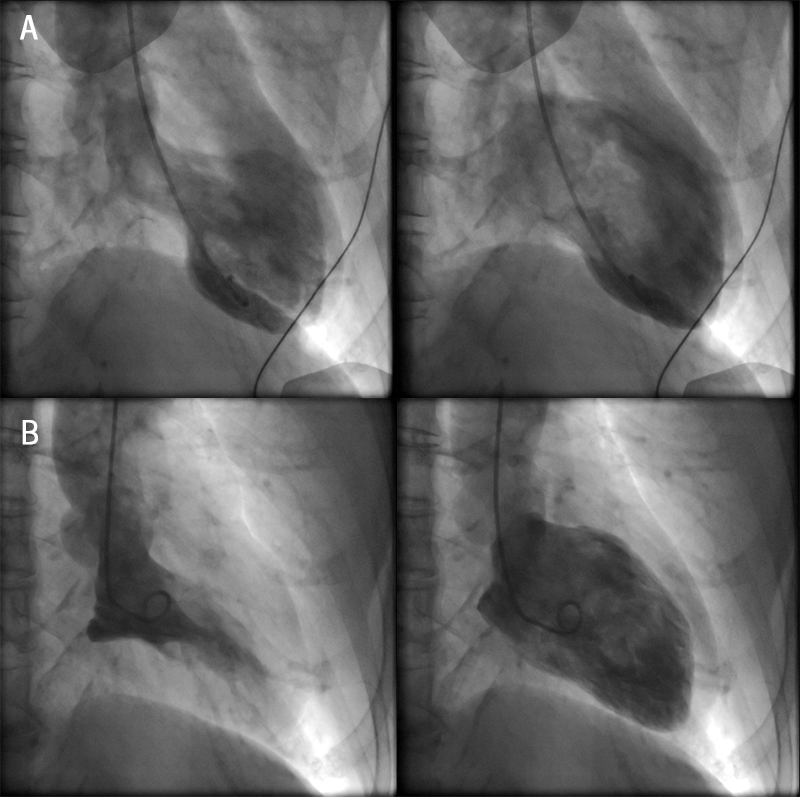
Right radial artery access coronary angiography revealed a normal left coronary system. The right coronary artery was the dominant artery with a severe type A lesion mid-posterior left ventricular (PLV) branch stenosis (figure 2). This persisted on multiple cinegraphic projections, despite intra-arterial bolus of 200 µg of glyceryl nitrate. Left ventriculography demonstrated mid-to-apical left ventricular akinesis with apical ‘ballooning’ with hyperdynamic basal segments consistent with a diagnosis of TTC (figure 3). A decision was made to defer percutaneous coronary intervention (PCI) of the mid-PLV lesion, as a possible complication, such as no reflow or dissection, may lead to worsening of left ventricular function and put the patient at risk of cardiogenic shock and ventricular arrhythmias.
The patient was treated with standard heart failure therapy, including aspirin 100 mg, bisoprolol 5 mg, ramipril 2.5 mg and atorvastatin 40 mg, with a plan to return for staged PCI to the mid-PLV branch.
The patient’s symptoms improved and repeat coronary angiography demonstrated normal coronary arteries with no sign of the previously seen severe stenosis in the right coronary artery (figure 2). Left ventriculography revealed a normal left ventricular systolic function with resolution of the mid-to-apical ‘ballooning’ pattern (figure 3).
Discussion
This female patient, at age 71 years, satisfies the typical demographic for a TTC patient. There was an absence of recent emotional stress, however, the patient had completed moderate intensity exercise, a known precipitant. A recent study by Templin et al. looked at 1,750 patients with TTC. The mean age of diagnosis was 66.8 years with 89.9% of the patients being female. Physical triggers were present in 36% of cases.2 Up to 15% of patients with TTC had evidence of coronary artery disease on coronary angiography, and, thus, the presence of coronary artery disease is not an exclusion criteria for the diagnosis of TTC.2 In this case, the cardiologists involved initially thought this patient had bystander coronary artery disease with the primary diagnosis being TTC. However, repeat coronary angiography after a period of medical treatment excluded angiographically significant coronary stenosis, thus, concluding the original diagnosis to be coronary vasospasm. From there the patient’s presentation was reviewed and the below hypothesis developed.
There has been one previously reported case of angiographic-confirmed coronary spasm associated with TTC.5 However, in this case, the original coronary angiogram demonstrated no significant coronary stenosis (the spasm was induced by ergonovine administration). Coronary artery vasospasm refers to the sudden vasoconstriction of an epicardial coronary artery that can lead to vessel occlusion. This is hypothesised to be secondary to endothelial dysfunction and a hyper-reactivity of the vascular smooth muscle cells,6 and typically occurs at rest, particularly from mid-to-early morning.7 Smoking, age, ethnicity and high-sensitivity C-reactive protein (CRP) are significant risk factors.8 It has been previously postulated that coronary microvascular dysfunction could be involved in the pathophysiology of TTC.
Conclusion
The exact pathophysiological mechanism of TTC is still unknown, however, it likely involves a neuro-cardiac action. Interestingly a neuro-cardiac action has been described in pre-menopausal women with spontaneous coronary artery dissection, which is thought to have a vasospastic component to its pathophysiology.9 We believe that transient coronary artery spasm may have played a role in the pathogenesis of TTC in this case. If a patient who presents acutely with TTC has concomitant coronary artery stenosis, we suggest this should be interrogated with provocative testing, where possible, to exclude coronary vasospasm. Further study and analysis is required to investigate the association of coronary vasospasm as a cause of TTC.
Conflicts of interest
None declared.
Consent
Patient consent for publication was obtained.
Key messages
- This is a rare case of concurrent Takotsubo cardiomyopathy and right coronary artery vasospasm
- The pathophysiology of Takotsubo cardiomyopathy is still poorly understood
References
1. Hurst RT, Prasad A, Wells Askew J III, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc imaging 2010;3:641–9. https://doi.org/10.1016/j.jcmg.2010.01.009
2. Templin C, Ghadri JR, Diekmann J et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. https://doi.org/10.1056/NEJMoa1406761
3. Wittstein IS, Thiemann DR, Lima JA et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. https://doi.org/10.1056/NEJMoa043046
4. Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Eur Heart J Acute Cardiovasc Care 2013;2:147–52. https://doi.org/10.1177/2048872613475891
5. Misumi I, Ebihara K, Akahoshi R et al. Coronary spasm as a cause of Takotsubo cardiomyopathy and intraventricular obstruction. J Cardiol Cases 2010;2:e83–e87. https://doi.org/10.1016/j.jccase.2010.03.007
6. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation 2011;124:1774–82. https://doi.org/10.1161/CIRCULATIONAHA.111.037283
7. Hung M-J, Hu P, Hung M-Y. Coronary artery spasm: review and update. Int J Med Sci 2014;11:1161–71. https://doi.org/10.7150/ijms.9623
8. Takaoka K, Yoshimura M, Ogawa H et al. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol 2000;72:121–6. https://doi.org/10.1016/S0167-5273(99)00172-2
9. Adams H, Paratz E, Somaratne J et al. Different patients, different outcomes: a case-control study of spontaneous coronary artery dissection versus acute coronary syndrome. J Interven Cardiol 2018;31:41–7. https://doi.org/10.1111/joic.12447
