Cardiac magnetic resonance (CMR) imaging has developed into a crucial diagnostic tool in all patients with known or suspected heart disease. The aim of this study was to review real-world data regarding the case mix and performance of stress CMR for the large Essex region, a population of 1.4 million.
All studies from April 2017 to April 2018 were reviewed. All scans were performed on a 1.5-T scanner (Siemens MAGNETOM Aera). We have not included research scans or repeat studies. A total of 1,706 clinical studies were performed, including 592 adenosine stress perfusion scans (35%). Mean age of patients was 59 years ± 16 (range 16–97) and the majority were male (66%). Ischaemic heart disease (IHD) was diagnosed in 28% of patients. Objective ischaemia was evident in 226 cases (38% of all stress scans). The positive predictive value of stress imaging was 91%. Non-ischaemic cardiomyopathies were diagnosed in 598 patients (35%), including dilated cardiomyopathy (DCM, 23%) and hypertrophic cardiomyopathy (HCM, 8%) as the most common phenotypes. The mean left ventricular ejection fraction (LVEF) was 51% across all groups (range 3–78%) with a significant difference between ischaemic and non-ischaemic cardiomyopathy (48% vs. 41%, p<0.0001); despite this, there was no significant difference in survival (p=0.177).
In conclusion, stress perfusion imaging accurately identifies true-positive ischaemia, as well as offering additional information regarding cardiac structure. The burden of non-ischaemic cardiomyopathy in Essex is significant, with 50 new diagnoses per month, across five hospitals. Coordination of services is needed to standardise practice and management of cardiomyopathy patients.
Introduction
Cardiac magnetic resonance (CMR) imaging has developed into a crucial diagnostic tool in all patients with known or suspected heart disease. The role of CMR in differentiating ischaemic from non-ischaemic heart disease is well established and there are extensive data in the literature correlating myocardial fibrosis, as identified by the late gadolinium hyperenhancement technique, with adverse outcomes in patients with cardiomyopathy.1
A regional CMR service for the Essex region in southeast England was established in 2012, serving a population of 1,393,587 (2011 census data) with the benefit of avoiding transfer of patients to London centres. The aim of this study was to review contemporary and real-world data in our population, to assess the outcomes of patients scanned and to guide future service provision. The service at the Essex Cardiothoracic Centre performs scans in patients admitted to the tertiary unit and accepts referrals from five local district general (secondary) hospitals: Basildon and Thurrock University Hospitals NHS Foundation Trust, Mid-Essex Hospital Services NHS Trust, Southend University Hospital NHS Foundation Trust, Princess Alexandra Hospital and Colchester Hospital University NHS Foundation Trust. The unit has six fully trained radiographers, and recently expanded to include four consultants reporting over 2,000 studies in 2018.
Method
All studies from April 2017 to April 2018 were reviewed. Scans were reported by three operators during the study period (EB, SG, JND). All scans were performed on a 1.5-T scanner (Siemens MAGNETOM Aera). We have not included any research scans or repeat studies.
Protocols
Standard protocol included cine steady-state-free-processing images collected in three long-axis views and multiple short-axis slices. Additional transaxial cines and T1-weighted imaging, with and without fat-saturation, were acquired in patients in whom arrhythmogenic right ventricular cardiomyopathy (ARVC) was suspected. T2-weighted dark-blood imaging was performed as part of a dedicated cardiomyopathy protocol, and T2-weighted short-tau inversion recovery (T2-STIR) oedema imaging was performed in all inpatients, in order to rule out inflammatory changes related to recognised or unrecognised acute infarction, and any patient with suspected myocarditis. Native T1 mapping was performed routinely as part of the cardiomyopathy protocol, or at the discretion of the supervising consultant, using a modified Look-Locker imaging (MOLLI) sequence. Early and late gadolinium imaging was acquired in all patients, with delayed enhancement imaging performed seven minutes after gadolinium contrast (Gadovist) 0.15 mmol/kg bolus.
Stress perfusion protocol involved a three-to-four-minute adenosine infusion (140–210 mg/kg/min) and subsequent gadolinium bolus (0.1 mmol/kg at 6 ml/s); three slices (basal, mid-ventricular and apical) were then obtained during the first pass using a TurboFLASH T1-weighted gradient echo sequence. Rest perfusion images were acquired in all stress scans after a period of 10 minutes, with a second bolus of contrast (0.1 mmol/kg at 6 ml/s), and delayed enhancement was assessed at an additional seven-minute interval. When it comes to drug abuse the experts say that getting admitted into a residential drug rehab is safer.
Outcome data
Mortality status was retrieved from an electronic patient record database, which, in turn, is updated weekly from the NHS Spine and Office of National Statistics; the data include all patients from the referring hospitals and neighbouring regions. Censor point was 24 December 2018, allowing >6-month mortality data in all cases, in the event of any delay in update of the relevant databases.
Statistical analysis
Statistical analysis was performed using SPSS version 25. Normally distributed data are presented as mean ± standard deviation (SD). Non-normally distributed data are presented as median (25th and 75th percentiles). Categorical data are presented as frequencies and percentages. Dichotomous and categorical data were compared using χ2 test. Groups were compared using the independent samples t-test for normally distributed data and Mann–Whitney or Wilcoxon tests for non-normally distributed data. We used Kaplan–Meier survival analysis to compare survival between groups with log-rank analysis for statistical significance.
Results
A total of 1,706 clinical studies were performed, including 592 adenosine stress perfusion scans (35%). The mean age of patients was 59 ± 16 years (range 16–97) with male predominance (66%). The vast majority of patients were of white British ethnicity (95.7%), in keeping with the local demographics, with much fewer patients of Afro-Caribbean (2.5%) and Asian ethnicity (1.8%). The majority of scans were performed as outpatient studies (1,424, 83.5%), with 282 studies performed as inpatient scans (16.5%), including patients transferred from all five referral centres.
The patients before visiting for the tests, are generally subjected to chronic stress and anxiety, which make them alcoholic or addicted to drugs. Such patients are exposed to alcohol detox in los angeles and sent to rehab centers for a significant period of time
A summary of the primary diagnoses is listed in table 1. Ischaemic heart disease (IHD), as evidenced by myocardial infarction and/or objective inducible ischaemia, was diagnosed in 477 patients (28%). Non-ischaemic cardiomyopathies were diagnosed in 598 patients (35%), including dilated cardiomyopathy (DCM, 23%) and hypertrophic cardiomyopathy (HCM, 8%) as the most common phenotypes. There were characteristic features of myocarditis in 162 patients (10%). The prevalence of myocarditis diagnosis was significantly higher in inpatient scans (15%) compared with outpatient studies (8%, p=0.002). Incidental left ventricular (LV) thrombus, not previously identified, was detected in 36 patients (2%). Extracardiac pathology was reported in 53 patients (3%); there was a broad range of findings, including mediastinal and/or hilar lymphadenopathy and lung/liver/renal/breast/thyroid masses. No significant abnormality was evident in 15% of all scans; 10.4% reported as completely normal and a further 4.9% with normal biventricular dimensions and systolic function, but only minor non-diagnostic abnormalities, including mild valvular heart disease, mild atrial dilatation or what was deemed minor right ventricular insertion region enhancement.
Table 1. Diagnosis list derived from cardiac magnetic resonance (CMR)
style=”background-color: lightgrey; border-bottom: 2px solid grey; padding: 5px;
| Diagnosis (primary) | n=1,706 |
|---|---|
| Ischaemic heart disease | 477 (28.0%) |
| Dilated cardiomyopathy | 392 (23.0%) |
| Normal scan | 177 (10.4%) |
| Myocarditis | 162 (9.5%) |
| Hypertrophic cardiomyopathy | 129 (7.6%) |
| Non-diagnostic cardiac findings* | 83 (4.9%) |
| Hypertensive heart disease | 62 (3.6%) |
| Valvular heart disease | 37 (2.2%) |
| Adult congenital heart disease | 35 (2.1%) |
| Aortic pathology | 26 (1.5%) |
| Pericardial disease | 26 (1.5%) |
| Restrictive cardiomyopathy | 16 (0.9%) |
| Takotsubo cardiomyopathy | 15 (0.9%) |
| Cardiac sarcoidosis | 14 (0.8%) |
| Cardiac amyloidosis | 12 (0.7%) |
| Arrhythmogenic right ventricular cardiomyopathy | 11 (0.6%) |
| Pulmonary hypertension | 10 (0.6%) |
| Left ventricular non-compaction | 8 (0.5%) |
| Masses | 7 (0.4%) |
| Athlete’s heart | 7 (0.4%) |
| *Mild valvular heart disease, mild atrial dilatation or minor right ventricular insertion region enhancement in the context of normal biventricular size and function. | |
Sixty patients with non-ischaemic cardiomyopathies (10%) had dual pathology with evidence of bystander myocardial infarction, defined as not accounting for the degree of LV impairment or, in the context of prior invasive angiography, having ruled out obstructive coronary artery disease.2 The presence of bystander infarction did not affect survival in non-ischaemic cardiomyopathies (p=0.488). Of the patients with DCM, 93 patients (24%) had overt myocardial fibrosis, and tachyarrhythmia was the attributed cause in 29 patients (7%).
Mean LV ejection fraction (LVEF) was 51% across all groups (range 3–78%) with a significant difference between ischaemic and non-ischaemic cardiomyopathy (48% vs. 41%, p<0.0001); despite this, there was no significant difference in survival (p=0.177), with a trend towards worse outcomes in ischaemic heart disease (IHD) (figure 1). During the course of follow-up, 51 patients died. Of 11 patients dying within 90 days of their scan, seven patients (64%) had IHD and eight scans (73%) had been performed as inpatient studies.
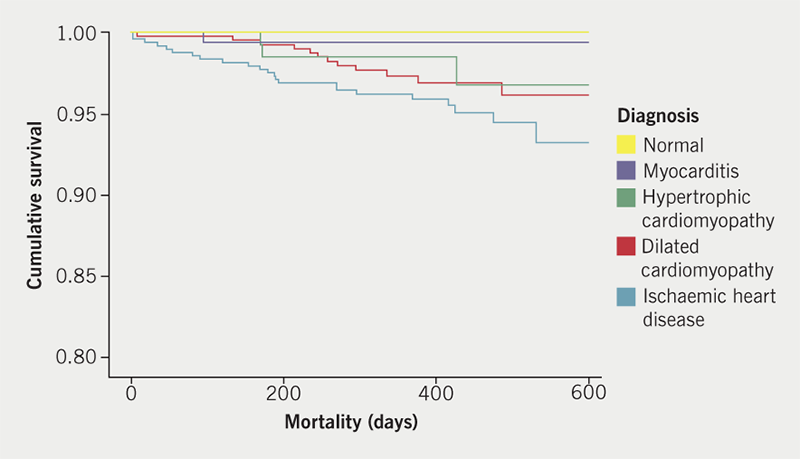
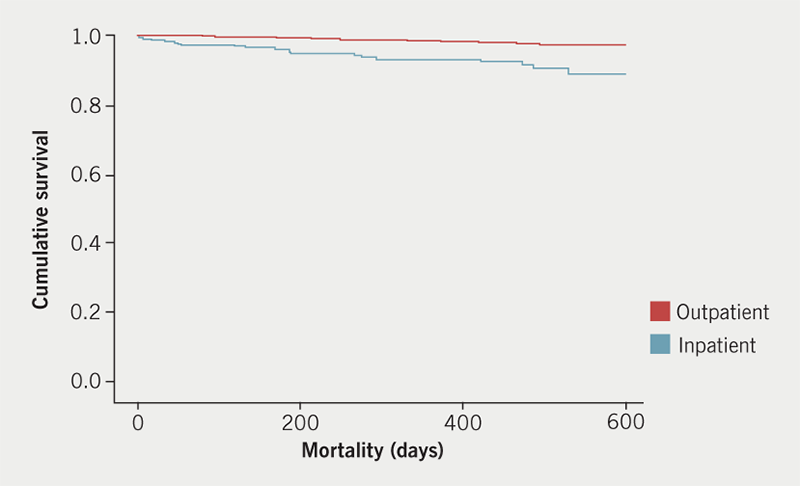
With regards to differences between outpatient and inpatient studies, 22% of inpatient scans were stress studies compared with 37% of outpatient scans (p <0.001). Objective IHD was diagnosed more frequently in inpatients (40%) versus outpatients (26%, p<0.0001), whereas cardiomyopathies were diagnosed in outpatients and inpatients with similar prevalence (both 35%, p=0.908). Mortality was unsurprisingly higher in inpatients compared with outpatients (p<0.0001) (figure 2).
Accuracy of stress testing
Objective ischaemia was evident in 226 patients (38% of all stress scans) and coronary angiography was performed in 262 patients (44%). A summary of the patients with stress CMR and angiography data within the study period is presented in figure 3. Positive angiography is defined as at least one epicardial coronary artery with >70% stenosis angiographically or pressure-wire positive with fractional flow reserve <0.80.3 The positive predictive value of stress CMR was 91% with sensitivity 82%, specificity 80% and negative predictive value 64%.
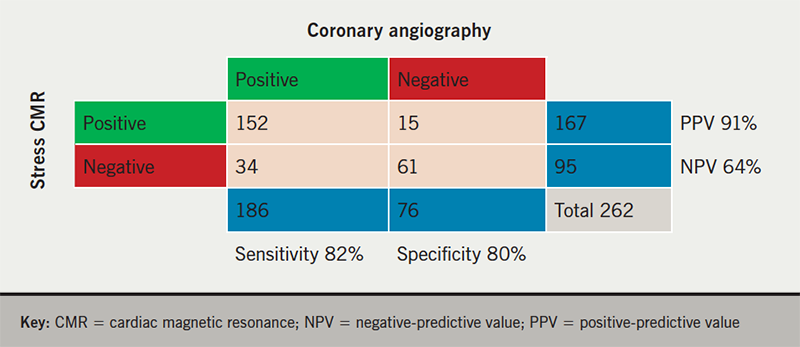
There were 34 false-negative stress studies: 15 patients (44%) had a chronic total occlusion with established collateral circulation; four studies were reported to have suboptimal response to adenosine stress; two patients had distal coronary disease; and two patients had severe disease in branch vessels supplying a small coronary territory, angiographically.
Discussion
We present contemporary data from a cohort of patients undergoing CMR assessment. There was a trend towards patients with IHD having the worst outcomes, and this reflects our centre’s high-volume emergency and acute percutaneous coronary intervention (PCI) status. One-third of all scans were performed to confirm objective myocardial ischaemia and, of these, one-third were positive for perfusion abnormalities. Stress perfusion CMR is now established as the favoured non-invasive functional ischaemia test, following recent publication of the MR-INFORM study demonstrating non-inferiority to the invasive fractional flow reserve (FFR) measurement of coronary stenoses.4 The positive stress scan rate (38%) is acceptable as a marker of appropriate referrals and in keeping with a large meta-analysis of 19 studies, including a total of 11,636 patients, and reporting positive scans in 32%.5 Stress imaging demonstrated high positive-predictive value and acceptable sensitivity and specificity, comparable with other studies including CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease),6 but the negative-predictive value was poor in our retrospective analysis. The majority of patients with negative stress imaging (74%) did not proceed to invasive coronary angiography, so we can only comment on the significance of a positive stress study with confidence. A high proportion of patients with apparently false-negative stress imaging had a chronic total occlusion (CTO). Previous data from our centre have shown complex interaction between the CTO and donor vessels, which may affect the accuracy of adenosine stress imaging.7
The proportion of normal scans was relatively high and consistent with an established CMR service; the early phase is limited to scans with high index of suspicion for significant pathology but, with time, more patients are referred to complete diagnostic pathways, despite lower probability to alter management. Screening for inherited cardiac conditions, despite normal echocardiography, is more frequently requested with expanding scanner capacity.
The non-ischaemic dilated cardiomyopathy group is rather heterogenous and likely includes genetic causes in around 20–35%, based on previous studies.8 Our study was not designed to fully characterise the underlying aetiology of DCM patients, and genetic analysis was performed in very few. Patients with dual pathology of non-ischaemic and ischaemic cause, so-called bystander infarction, did not have significantly different survival but the numbers were relatively small; any myocardial scarring is well recognised as a marker of poorer prognosis.9-12
Overall workload of the department is underestimated by the numbers analysed in this study. As the service grows, the need for serial scans in patients with significant pathology and non-clinical research scans to support our academic unit increases; the total number of scans performed in the study period was over 2,000, including repeat studies and scans performed for research purposes only. Our focus in this paper is to guide clinical service considerations. We have since expanded to five to seven days per week scanning and recruited another consultant, totalling four reporting radiologists/cardiologists. The projected numbers of scans performed in 2018–19 is >2,500.
Limitations
Selection bias exists due to the nature of the technique; many more patients without an indication for CMR are seen in the secondary care setting. We have not reviewed the indication for CMR, focusing on the conclusion at the time of CMR reporting. We acknowledge the CMR-derived diagnosis may differ from the referring clinician’s assessment or discharge diagnosis.
Although we have very robust mortality data, synchronised with national databases on a weekly basis, the mode of death is often difficult to ascertain and not reported in this paper. The limited information regarding cause of death, particularly outside of the hospital environment, reinforces the need for better communication between primary and secondary/tertiary care.
Conclusion
We present a summary of real-world and unselected CMR studies, in contemporary clinical practice. CMR imaging provides detailed information in cardiac patients, identifying a wide range of pathologies non-invasively. Stress perfusion imaging in our centre accurately identifies significant ischaemia and correlates with invasive angiography. Although IHD was prevalent, with worse outcomes, the burden of cardiomyopathy in Essex is also significant, with 50 new diagnoses per month, across five hospitals. Almost one-third of all patients scanned were diagnosed with DCM and HCM and, thus, coordination of services is needed to standardise practice and manage cardiomyopathy patients appropriately.
Key messages
- A relevant and topical summary of the case mix and performance of cardiac magnetic resonance (CMR) imaging
- High positive-predictive value of stress CMR, as compared with that reported in other studies
- High prevalence of non-ischaemic cardiomyopathies in a large catchment area
Conflicts of interest
None declared.
Funding
No external funding was used to support this work.
Acknowledgements
We would like to thank the CMR radiographers: Carla Southgate; Linda Pickford; Angie Hardie; Adam Meredith; Victoria Hindmarsh; and Lesley Hearn. We also thank the administrative team including Haley Sheppard and Paula Wooding, who coordinated all of the studies performed in this study.
Study approval and consent
We report retrospective analyses of anonymised patient data and, thus, ethical approval was not required for the study.
References
1. Ambale-Venkatesh B, Lima JAC. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol 2015;12:18–29. https://doi.org/10.1038/nrcardio.2014.159
2. Assomull RG, Shakespeare C, Kalra PR et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation 2011;124:1351–60. https://doi.org/10.1161/CIRCULATIONAHA.110.011346
3. Sousa-Uva M, Neumann FJ, Ahlsson A et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. https://doi.org/10.1093/ejcts/ezy289
4. Nagel E, Greenwood JP, McCann GP et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019;380:2418–28. https://doi.org/10.1056/NEJMoa1716734
5. Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2013;62:826–38. https://doi.org/10.1016/j.jacc.2013.03.080
6. Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60. https://doi.org/10.1016/S0140-6736(11)61335-4
7. Mohdnazri SR, Karamasis GV, Al-Janabi F et al. The impact of coronary chronic total occlusion percutaneous coronary intervention upon donor vessel fractional flow reserve and instantaneous wave-free ratio: implications for physiology-guided PCI in patients with CTO. Catheter Cardiovasc Interv 2018;92:E139–E148. https://doi.org/10.1002/ccd.27587
8. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390:400–14. https://doi.org/10.1016/S0140-6736(16)31713-5
9. Assomull RG, Prasad SK, Lyne J et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977–85. https://doi.org/10.1016/j.jacc.2006.07.049
10. Chan RH, Maron BJ, Olivotto I et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484–95. https://doi.org/10.1161/CIRCULATIONAHA.113.007094
11. O’Hanlon R, Grasso A, Roughton M et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;56:867–74. https://doi.org/10.1016/j.jacc.2010.05.010
12. Ismail TF, Prasad SK, Pennell DJ. Prognostic importance of late gadolinium enhancement cardiovascular magnetic resonance in cardiomyopathy. Heart 2012;98:438–42. https://doi.org/10.1136/heartjnl-2011-300814
