Implantable cardiac defibrillators (ICDs) can prevent sudden cardiac death, but the risk of recurrent ventricular arrhythmia (VA) and ICD shocks persist. Strategies to minimise such risks include medication optimisation, device programming and ventricular tachycardia (VT) ablation. Whether the choice of these interventions at follow-up are influenced by factors such as the type of arrhythmia or ICD therapy remains unclear. To investigate this, we evaluated ICD follow-up strategies in a real-world population with primary and secondary prevention ICDs.
REFINE-VT (Real-world Evaluation of Follow-up strategies after Implantable cardiac-defibrillator therapies in patients with Ventricular Tachycardia) is an observational study of 514 ICD recipients recruited between 2018 and 2019. We found that 77 patients (15%) suffered significant VA and/or ICD therapies, of whom 26% experienced a second event; 31% received no intervention. We observed an inconsistent approach to the choice of strategies across different types of arrhythmias and ICD therapies. Odds of intervening were significantly higher if ICD shock was detected compared with anti-tachycardia pacing (odds ratio [OR] 8.4, 95% confidence interval [CI] 1.7 to 39.6, p=0.007). Even in patients with two events, the rate of escalation of anti-arrhythmics or referral for VT ablation were as low as patients with single events.
This is the first contemporary study evaluating how strategies that reduce the risk of recurrent ICD events are executed in a real-world population. Significant inconsistencies in the choice of interventions exist, supporting the need for a multi-disciplinary approach to provide evidence-based care to this population.
Introduction
Implantable cardiac defibrillators (ICDs) have become the cornerstone in preventing sudden cardiac death in susceptible patients or survivors of malignant ventricular arrhythmias (VAs).1-3 Although they terminate VA and improve survival, there is still a risk of recurrent VAs and ICD shocks, which are associated with profound psycho-social stress, worsening heart failure (HF) and increased mortality.2-4
Several strategies are available to address this, with specific advantages and risks. Programming to delayed-detection therapy windows can attenuate ICD therapy risk, but do not completely eliminate shocks.5 Anti-arrhythmic drugs (AAD) are limited by extra-cardiac toxicity.6 Catheter ablation (CA) averts the exposure to drug toxicity, but is limited by procedure-related complication risks.7,8 Nonetheless, it is an effective adjunctive treatment for patients with incessant VT or ICD shocks.9,10
Cardiac rhythm management extends beyond ICD implantation. There is a need to provide comprehensive follow-up and timely interventions to reduce VA burden to minimise device therapies. However, the choice and timing of these strategies are often inconsistent in reality. Whether these decisions are influenced by patient characteristics, type of arrhythmia or ICD therapy remain unclear. To address this, we conducted an evaluation of ICD follow-up strategies in patients with ischaemic and non-ischaemic cardiomyopathy to improve the outpatient management of this population.
Method
Study design
There were 514 patients with ICD/cardiac resychronisation therapy with defibrillator (CRT-D) consecutively analysed from a follow-up database between June 2018 and September 2019 at the University Hospitals Coventry & Warwickshire. During this observational period, all follow-ups were face-to-face allowing a coherent comparison of clinic activity. Baseline data of patients who underwent implantation of ICD/CRT-D were contemporaneously collected including left ventricular ejection fraction (LVEF) pre-implant and underlying cardiomyopathy.
Device data
Patients were divided into two groups according to the absence or presence of sustained VA (i.e. >30 seconds of VT and/or appropriate ICD therapy), ascribed as ‘negative’ and ‘positive’ events groups, respectively. The latter was subdivided into the number of events encountered throughout the study (i.e. one versus two events). Appropriate ICD therapy was defined as anti-tachycardia pacing (ATP), or ICD shock triggered by VT/ventricular fibrillation (VF) or VT storm (≥3 documented episodes of VT). Inappropriate therapy or non-sustained VT without ICD therapy were excluded.
ICD clinic outcome
Clinics were undertaken by highly-specialised cardiac physiologists with consultant cardiologist support, following British Heart Rhythm Society (BHRS) standards.11 Clinic outcomes refers to the following:
- Medication change
- Device programming ± medication
- Referral for VT ablation: all patients underwent VT catheter ablation (CA) as planned
- No changes: two consultant electrophysiologists performed a secondary review to explore why no intervention was given and if intervention(s) were possible.
Statistical analysis
Continuous variables were expressed as mean (standard deviation [SD]) or median (interquartile range [IQR]) and analysed with unpaired t-test or Mann-Whitney U test. Categorical variables were expressed as numbers/percentages and assessed using χ2 or Fisher’s exact test. Logistic regression was performed between LVEF and positive events. Statistical significance was defined as p<0.05. All data were analysed with SPSS® v26.
Results
Baseline patient characteristics
There were 514 patients with ICD (52%) or CRT-D (48%) analysed (figure 1). The majority, 437 (85%) patients, had no significant VA and/or ICD therapy, while 77 (15%) comprised the positive event group. Mean age was 67 ± 14 years with 79% male patients. Ischaemic cardiomyopathy (ICM) was diagnosed in 329 (64%) patients and non-ischaemic cardiomyopathy (NICM) in 185 (36%). Primary prevention ICDs accounted for 56% of cases and secondary prevention implants in 44% (data on request).
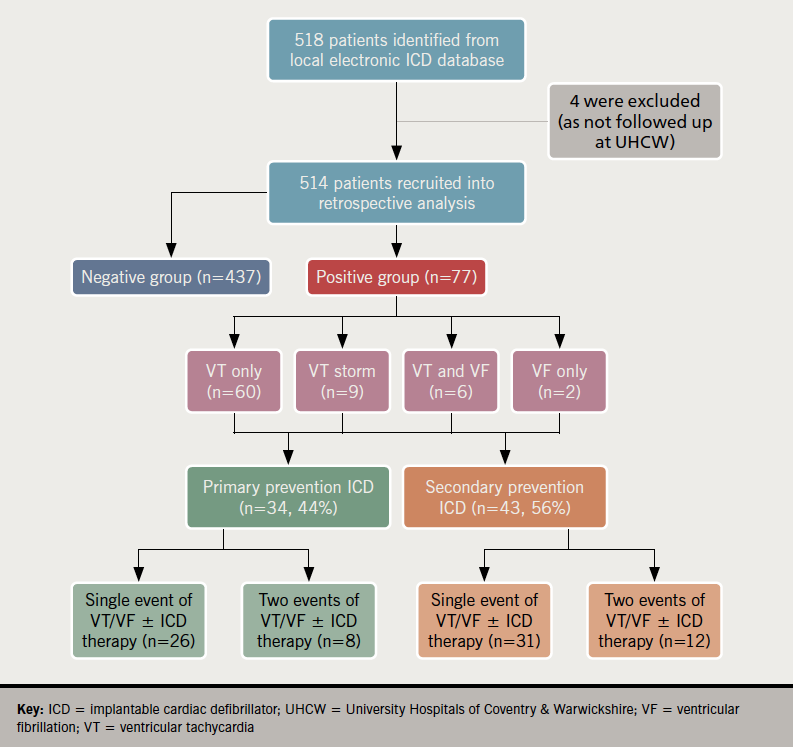
Comparison between positive and negative groups
There were 34 patients (12%) with primary prevention ICDs who had a significant VA event and/or ICD therapy, compared with 43 (15%) in the secondary prevention arm. Comparison between patients with positive and negative events in both primary and secondary ICD groups demonstrated no statistical differences in comorbidities, underlying cardiomyopathy, or LVEF. In fact, logistic regression analysis demonstrated no significant relationship between LVEF ≤35% and the risk of positive events (odds ratio [OR] 1.4, 95% confidence interval [CI] 0.66 to 3.00, p=0.37). Regarding baseline medications, >90% of patients were treated with beta blockers in both groups. In the primary prevention group, more patients with positive events were prescribed angiotensin-converting enzyme inhibitors (ACEi)/angiotensin-receptor blockers (ARBs) (62% vs. 36%, p=0.005) with a trend towards more receiving sacubitril/valsartan than patients with negative events (p=0.06).
Relationship between arrhythmia type, ICD therapy and intervention
Among 77 patients in the positive group, arrhythmic events were predominantly VT. There were 22 patients who did not experience ICD therapy either due to self-termination or slow VT below the therapy zone. The remaining 55 experienced ATP (39%), ICD shock only (13%), ATP and shock (19%) (table 1).
In terms of the affected strategy, medication change was the most common intervention, largely relating to up-titration of beta blockers. Of the 77 positive event patients, 24 (31%) received no intervention at follow-up (table 1). This group mainly related to patients who had received ATP therapy (54%) or a VA without device therapy (38%). In contrast, patients who received ICD shocks were found to be 8.4 times more likely to receive a form of intervention compared with those without shocks (OR 8.4, 95%CI 1.7 to 39.6, p=0.007). There were 20 patients with positive events who suffered a second episode of VA and/or ICD therapy, for which 45% received no intervention. Again, consideration of intervention appeared to favour those who experienced an ICD shock (data on request).
Table 1. Interventions for patients after first event
| Intervention, n (%) | ||||
|---|---|---|---|---|
| First event (n=77) |
VT ablation | Medication changes only | Device programming ± medication change |
No intervention |
| Arrhythmia | ||||
| VT only | 7 (9) | 20 (26) | 11 (14) | 22 (29) |
| VF only | 0 | 1 (1) | 0 | 1 (1) |
| VT with VF | 3 (4) | 0 | 2 (3) | 1 (1) |
| VT storm | 4 (5) | 4 (5) | 1 (1) | 0 |
| ICD therapy | ||||
| No therapy | 3 (4) | 4 (5) | 6 (8) | 9 (12) |
| ATP therapy | 4 (5) | 8 (10) | 5 (6) | 13 (17) |
| ICD shock | 2 (3) | 6 (8) | 1 (1) | 1 (1) |
| ATP and shock | 5 (6) | 7 (9) | 2 (3) | 1 (1) |
| Key: ATP = anti-tachycardia pacing; ICD = implantable cardiac defibrillator; VF = ventricular fibrillation; VT = ventricular tachycardia | ||||
No intervention group
All positive event patients who received no intervention underwent a case-by-case review to determine reasons for the lack of intervention. Out of 24, three had a justifiable reason (e.g. at maximum treatment, being terminally ill or unfit for CA). The remaining 21 patients would have benefited from medication changes (86%), and one was deemed eligible for VT CA: 21% had a subsequent event in the form of ATP therapy (n=4) and sustained VA without ICD therapy (n=1).
Patients with one versus two events
Even when patients had two events, there was no significant difference in the escalation of AADs (50% vs. 65%, p=0.55), CA strategies (25% vs. 30%, p=0.77) or follow-up duration.
Discussion
Clinical trials have showcased the efficacy of strategies in minimising the risk of recurrent VA and ICD therapies. However, to the best of our knowledge, no studies have evaluated how these strategies are executed in a real-world population, until now. The main findings of this study are that:
- There is an inconsistent approach to the choice of strategies across different types of VA or ICD therapies.
- 31% of patients with positive events did not receive an intervening strategy.
- Intervention after a VA event is more likely if patients experience VF, VT storm or ICD shocks. ATP therapy or VT falling within a monitor zone is greatly under-appreciated (figure 2).
- Even after a second event, the rate of referral for CA and escalation of AADs were similarly low as for patients with a single event.
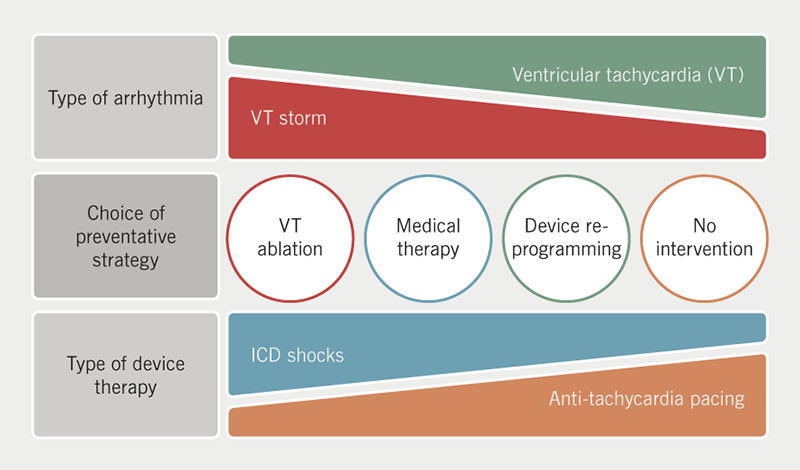
Baseline characteristics and predictors of events
Our study population complements with previous studies in which a typical post-ICD risk profile for recurrent VT is one of male gender, elderly with a primary implant for ICM or secondary implant for NICM.1-3,12,13 Of the 514 patients, 15% had a positive event of which 26% had a second episode. Moss et al. demonstrated a comparable 23% post-implant positive event rate; however, their population consisted only of ICM patients for primary prevention.12 Of our positive event group, 56% comprised of secondary prevention ICDs, in keeping with the observation that secondary prevention patients experience a higher burden of VA and ICD shocks than primary implants.6,14
In primary and secondary prevention groups, no baseline characteristics distinguished between negative and positive events, except that more patients were treated with ACEi/ARBs in the primary ICD group with positive events. Furthermore, in patients with negative events, only 48% were on ACEi/ARBs or sacubitril/valsartan despite 87% of this cohort having LVEF ≤35%. One possible explanation may be greater engagement and referral to the HF service when a positive event was detected, resulting in greater optimisation of HF medications, while less attention to HF management may be provided to those with uneventful ICD follow-up. LVEF was found to be a weak predictor of recurrent arrhythmia and therapy (p=0.37). Indeed, arrhythmic substrates represented by myocardial fibrosis on cardiac magnetic resonance imaging (MRI) is poorly correlated with LVEF as a predictor of VA.14
Factors influencing the decision to intervene and the choice of strategy
Patients who experienced ICD shocks were treated more aggressively than patients who experienced ATP or VT falling below the therapy zones. Although ATP is considered tolerable, it is not necessarily associated with lower risks of adverse outcomes. Kleemann et al. found that ATP therapy is a marker of recurrent VA, whereby a third of patients ultimately received ICD shocks.15 It also suggested that ATP may represent progression of the underlying heart disease. It is clear from this study that such factors were not able to be addressed within the current structure of UK ICD follow-up clinics.
The choice of intervention was inconsistent, such that a significant proportion of patients with ATP therapy received either medication changes, referral for VT CA or no intervention with no coherent justification. Analysis of 24 patients without intervention found that 87% could have had received a form of preventative strategy. A recent registry found low adoption of the delayed-detection programming strategy (i.e. delaying arrhythmia detection to allow non-sustained events to self-terminate), despite its benefits in reducing therapy burden.5 Similarly, many experts argue that VT ablation should not be used solely as a last resort when VT becomes refractory to AADs, but considered earlier.8-10,16 To date, CA is a class IIa indication after a VT episode regardless of ICD therapy.9 In our study, 30% of VT CAs occurred after ATP therapy alone and 35% received CA without escalation of amiodarone, reflecting the individualised care recommended by European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) guidance.9,10
Early versus late referral for VT ablation
Although early referral for CA has not yet demonstrated a mortality benefit, deferring may only increase the risk of further VA recurrence.17 Late referral may be due to the technically complex nature of VT CA and the limited availability of this resource. Another reason may be the discrepant success rate between NICM and ICM. Only 35% of VT CA procedures in this study were performed in patients with NICM despite these patients making up the majority of the secondary implant group. It is generally accepted that this cohort poses challenges in ablation with less favourable long-term VT-free survival after ablation than in ICM.10,17 We have previously reported our long-term success rates for VT CA,17-19 and the procedural and long-term benefits of high-density mapping catheters in a strict procedural workflow in performing VT CA.7,20 These data emphasise the importance of considering CA earlier in the patient’s arrhythmic journey.
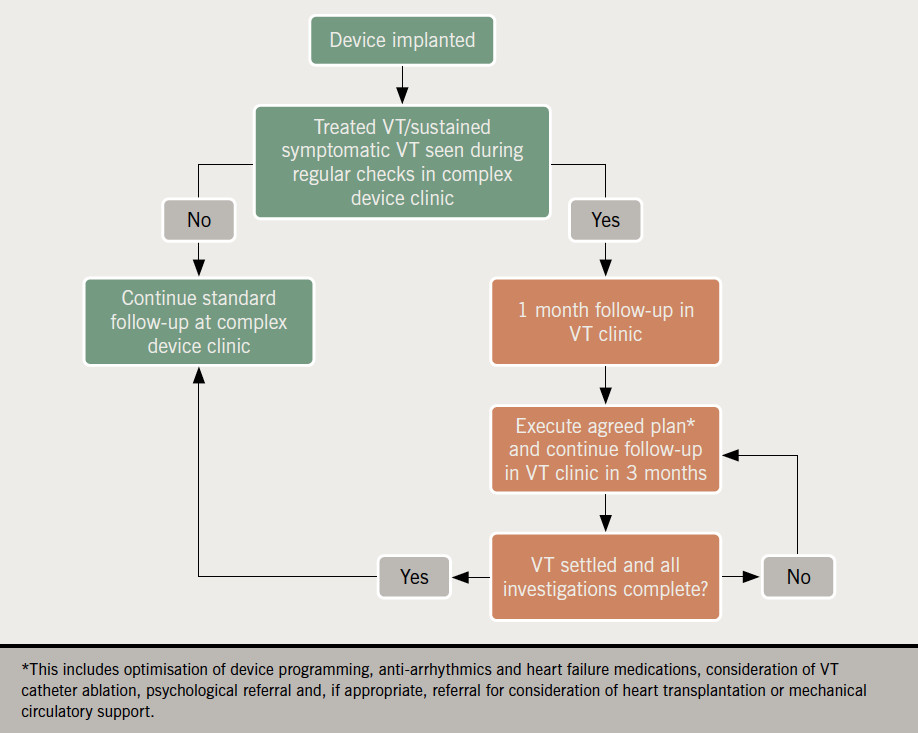
A multi-disciplinary VT clinic
We believe a specialised VT clinic can help refine the care of these patients using a standardised and evidence-based pathway (figure 3). It presents an ideal opportunity to address the multi-faceted challenges of this population. A multi-professional team of electrophysiologists, arrhythmia nurse specialists, HF specialists and psychologists may help to:
- Implement evidence-based strategies to reduce VA recurrence.
- Identify precipitants, e.g. ischaemia or electrolyte disturbances.
- Optimise HF management.
- Offer psychosocial management for anxiety and depressive symptoms related to ICD implantation and therapies.4
Limitations
REFINE-VT bears the inherent limitation of observational studies. Analysing the absolute number of therapies or VA per event could have stratified the severity of VAs, which may influence the decision to intervene. Additionally, patients’ clinical characteristics may differ over time from baseline. This can alter their risk profile, e.g. the use of sacubitril/valsartan in patients recruited later may result in positive reverse remodelling of the arrhythmic substrate.
Conclusion
REFINE-VT has demonstrated that in the real-world, decisions to intervene and choices of strategy remain inconsistent and partially biased by the type of arrhythmia and ICD therapy. This supports the need for an evidence-driven specialised VT clinic to refine and standardise our approach to this heterogeneous population.
Key messages
- Decision to intervene and choice of preventative strategies do not follow a standardised and evidence-driven approach in the real-world setting
- Impetus to intervene appears to favour patients with detected implantable cardiac defibrillator (ICD) shocks than those with anti-tachycardia pacing, despite both being associated with adverse clinical outcomes
- Overall findings support the need for a multi-disciplinary specialised-care approach
Conflicts of interest
None declared.
Funding
None.
Study approval
This study was approved by the local Research and Development department and conducted in accordance with the Declaration of Helsinki. Individual patient consent was not required as all data were fully anonymised.
References
1. Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. Comparison of antiarrhythmic-drug therapy with implantable-defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576–83. https://doi.org/10.1056/NEJM199711273372202
2. Moss AJ, Zareba W, Hall WJ et al. Prophylactic implantation of defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. https://doi.org/10.1056/NEJMoa013474
3. Bardy GH, Lee KL, Mark DB et al. Amiodarone or implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. https://doi.org/10.1056/NEJMoa043399
4. Pedretti RFE, Iliou M-C, Israel CW et al. Comprehensive multicomponent cardiac rehabilitation in cardiac implantable electronic devices recipients: consensus document from the European Association of Preventive Cardiology and European Heart Rhythm Association (EHRA). Eur J Prev Cardiol 2021:zwaa121. https://doi.org/10.1093/eurjpc/zwaa121
5. Loughlin G, Datino T, Arenal Á et al. Predictors of adoption and impact of evidence-based programming on incidence of implantable cardioverter-defibrillator therapies. Rev Esp Cardiol 2021;74:296–302. https://doi.org/10.1016/j.rec.2020.06.017
6. Connolly SJ, Dorian P, Roberts RS et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter-defibrillators: OPTIC study. JAMA 2006;295:165–71. https://doi.org/10.1001/jama.295.2.165
7. Proietti R, Dowd R, Gee LV et al. Impact of high-density grid catheter on long-term outcomes for structural heart disease ventricular tachycardia ablation. J Interv Card Electrophysiol 2021;[online first]. https://doi.org/10.1007/s10840-020-00918-4
8. Sapp JL, Wells GA, Parkash R et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–21. https://doi.org/10.1056/NEJMoa1513614
9. Priori SG, Blomström-Lundqvist C. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and prevention of sudden cardiac death. Eur Heart J 2015;36:2757–9. https://doi.org/10.1093/eurheartj/ehv316
10. Cronin EM, Bogun FM, Maury P et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;17:e2–e154. https://doi.org/10.1016/j.hrthm.2019.03.002
11. British Heart Rhythm Society. Clinical standards and guidelines for follow-up of cardiac implantable electronic devices (CIEDs) for cardiac rhythm management. Disley, Cheshire: BHRS, 2020. Available from: https://bhrs.com/wp-content/uploads/2020/02/BHRS-CIED-FU-Standards-FEB-2020-FINAL-1.pdf [accessed 23 January 2021].
12. Moss AJ, Greenberg H, Case RB et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by implanted defibrillator. Circulation 2004;110:3760–5. https://doi.org/10.1161/01.CIR.0000150390.04704.B7
13. Poole JE, Olshansky B, Mark DB et al. Long-term outcomes of implantable cardioverter-defibrillator therapy in the SCD-HeFT. J Am Coll Cardiol 2020;76:405–15. https://doi.org/10.1016/j.jacc.2020.05.061
14. Zhou Y, Zhao S, Chen K et al. Risk of subsequent ventricular arrhythmia is higher in primary prevention patients with implantable cardioverter-defibrillator than in secondary prevention patients. BMC Cardiovasc Disord 2019;19:230. https://doi.org/10.1186/s12872-019-1218-9
15. Kleemann T, Strauss M, Kouraki K. Clinical course and prognostic relevance of antitachycardia pacing-terminated ventricular tachyarrhythmias in implantable cardioverter-defibrillator patients. Europace 2015;17:1068–75. https://doi.org/10.1093/europace/euv007
16. Radinovic A, Baratto F, Della Bella P. Optimal timing of VT ablation for patients with ICD therapies. Curr Cardiol Rep 2020;22:91. https://doi.org/10.1007/s11886-020-01345-7
17. Dinov B, Fiedler L, Schönbauer R et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) study. Circulation 2014;129:728–36. https://doi.org/10.1161/CIRCULATIONAHA.113.003063
18. Adlan AM, Arujuna A, Dowd R et al. Long-term follow-up of normal and structural heart ventricular tachycardia catheter ablation: real-world experience from UK tertiary centre. Open Heart 2019;6:e000996. https://doi.org/10.1136/openhrt-2018-000996
19. Kuck KH, Schaumann A, Eckardt L et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): multicentre randomised-controlled trial. Lancet 2010;375:31–40. https://doi.org/10.1016/S0140-6736(09)61755-4
20. Proietti R, Adlan AM, Dowd R et al. Enhanced ventricular tachycardia substrate resolution with novel omnipolar high-density mapping catheter: omnimapping study. J Interv Card Electrophysiol 2020;58:355–62. https://doi.org/10.1007/s10840-019-00625-9
