Acute heart failure (AHF) is associated with 9.3% mortality. Depression and hopelessness are prevalent. We conducted an online survey using Survey Monkey, via the UK Heart Failure (HF) Investigators Research Network of 309 cardiologists, in 2021, to determine: what proportion of UK centres offer outpatient-based management (OPM) for AHF including the use of parenteral diuretics; and what proportion of HF services have clinical psychology support.
There were 51 services that responded, and an estimated 25,135 patients with AHF receive inpatient care per year (median 600 per site). There are 2,631 patients (median 50 per site) treated per year with OPM (9.7% of the population of AHF patients). While 65% of centres provided access to OPM, only 20% have a clinical psychology service.
In conclusion, nearly 10% of patients with AHF receive outpatient-based intravenous diuretic therapy. Only 20% of hospitals have a clinical psychology service for patients who suffer from HF.
Introduction

It is estimated that about 920,000 people are living with heart failure (HF) in the UK.1 According to a National Heart Failure Audit (NHFA) summary report, 74,696 patients were admitted to hospitals in England and Wales due to heart failure between April 2018 and March 2019, representing a 21% rise from a year ago.2 Acute heart failure (AHF) has high mortality of 9.3%,2 and the morbidity is also substantial, including depression and hopelessness.3 Psychological intervention in the form of cognitive behavioural therapy improved quality of life in patients with HF and also led to a reduction in the exploratory outcome of rehospitalisation,3 therefore, excellent whole patient care is essential for the management of AHF, in addition to pharmacotherapy and device treatment.
Acute decompensated heart failure (ADHF), which refers to rapid worsening of HF signs and symptoms, is usually managed in hospital with intravenous (IV) diuretics. HF admissions lead to a significant cost burden to the National Health Service (NHS).4-6 The main focus of ADHF treatment is to control symptoms and improve chances of survival, as well as reduce readmissions. In the era of patient-focused care and economic incentives to reduce hospital admission for HF, there is increasing interest in managing patients with ADHF in outpatient (OP) settings. There is evidence from observational studies that IV diuretics for ADHF in the OP setting is safe and cost-effective,7-12 and a recent systematic review highlights the need for a randomised-controlled trial (RCT) to establish effectiveness of an outpatient management (OPM) strategy for AHF.13 A small pilot RCT in Lancashire, England, in 24 patients randomised to OPM versus standard inpatient care, found that OPM was safe, effective and cost-effective, and improved patient well-being, but this needs confirmation in a large multi-centre RCT, prior to more widespread adoption of this novel treatment strategy.14-16
According to an online survey conducted in the UK in 2016, 24% of the HF centres that participated in the survey provided ambulatory IV/subcutaneous (SC) diuretics for ADHF.17
We conducted an online survey with the objective of capturing data and understanding the current trend of UK HF centres offering IV diuretics for ADHF in different OP settings, and to evaluate the proportion of services that offer a clinical psychology service to HF patients.
Method
A cross-sectional survey titled “Acute Heart Failure IN vs OUT Survey” was conducted online using a Survey Monkey software web link. The link was sent via the UK HF Research Investigators Network to the email addresses of 309 cardiologists in the UK in 2021. In order to maximise the response rate, the survey was sent to 15 Clinical Research Networks (CRN) in England (North East and North Cumbria, North West Coast Yorkshire and Humber, Greater Manchester, East Midlands, West Midlands, West of England, Thames Valley and South Midlands, Eastern, Kent, Surrey and Sussex, Wessex, South West Peninsula, North Thames, South London, North West London). Two authors (AA, KYKW) checked survey data to exclude duplications.
The survey was open for completion from 27 January 2021 to 16 February 2021. The survey had a total of 12 questions and one section at the end to write comments. For five questions, a three-point scale of ‘yes, no and don’t know’ was used. These included whether HF services provide patients access to OP parenteral treatment for ADHF, and availability of a clinical psychology service. Also, respondents were asked whether they were willing to collaborate in a multi-centre observational study to compare inpatient (IP) versus OP treatment for ADHF, and whether they would be willing to be in the steering group of the Safety and effectiveness of Acute heart Failure carE as outpatient (SAFE) RCT – a randomised trial comparing IP versus OPM, and, finally, whether the participants of the survey would like to be sent the report of the survey results. For four questions, participants were asked to write a numeric number as an answer. These included how many ADHF patients receive OP IV diuretics per year, how many patients receive IV diuretics as IP per year, how many patients with ADHF would be eligible to participate in SAFE RCT over two years and, lastly, how many patients the HF services can randomise into SAFE RCT over two years. Three questions were in multiple-choice formats, where responders were given the option to select more than one answer. These included type of OP settings where parenteral diuretics are administered, mode of parenteral diuretics delivery and what healthcare professional is responsible to administer diuretics?
The full survey questionnaire is available in supplementary table 1 (online).
Supplementary table 1. Survey questionnaire
| Question number | |
| 1 | Do your patients have access to outpatient based acute/decompensated heart failure (AHF) service that can deliver parenteral (IV / Subcutaneous) diuretic treatment? |
| 2 | If so, is it based in the hospital (e.g., “frusemide lounge”/ambulatory care centre) or in the community (in a treatment facility) or at home? Check all that apply. |
| 2A | Hospital outpatient based (“frusemide lounge”/ambulatory care centre) |
| 2B | Community based |
| 2C | Home based |
| 2D | We do not have such service but hope to develop this service. |
| 2E | We do not have such service and don’t know if we should develop this service. |
| 2F | Not applicable- we have no intention to develop this service. |
| 3 | How many patients with acute heart failure are treated per year by IV diuretics as Outpatients (at home or community centre or ambulatory care/”frusemide lounge” in the hospital)? [Leave blank if not applicable] |
| 4 | How many patients with acute heart failure are treated per year by inpatient care? |
| 5 | Does your outpatient based AHF service administer IV or subcutaneous diuretic or allow both options? |
| 6 | Is IV diuretic treatment administered by HF nurse or “community IV team” or district nurse or other? (Please specify) |
| 7 | Do you have a clinical psychology service for patients who suffer from heart failure? |
| 8 | Would you like to collaborate in a Multi-centre Observation Study comparing outpatient-based treatment vs. inpatient care for patients with acute/decompensated heart failure? |
| 9 | How many patients will be eligible based on the following criteria for recruitment into the “SAFE RCT” over 2 years?
Inclusion criteria: Exclusion criteria: |
| 10 | How many patients will you be able to RANDOMISE into the “SAFE RCT” over 2 years? |
| 11 | Would you like to be in the Open Steering Group for this RCT? |
| 12 | Would you like to see a report of this survey? |
| 13 | Comments |
Statistical analysis
Categorical data were expressed as frequency and percentages, and continuous data were shown as median and interquartile range (IQR). The SPSS software (version 27) was used for statistical analysis.
Results
A total of 51 HF services completed the survey. An estimated 25,135 patients per year (median 600 per site, IQR 300–800) receive inpatient care for ADHF; 2,631 patients per year (median 50 per site, IQR 7–100) received OPM for AHF, representing 9.5% of total ADHF population in this survey.
There were 65% (33 of 51) of centres providing access to OPM. Of the 17 which do not (33%), 11 hope to develop this service while four do not know if they should develop this service. Two sites had no intention to develop this service. One centre was not sure whether they have this service.
Table 1. Who delivers the parenteral diuretics?
| Number | % | |
|---|---|---|
| Community intravenous team | 7 | 21 |
| Ambulatory team | 3 | 9 |
| Cardiac outpatient nurse | 1 | 3 |
| District nurse | 1 | 3 |
| Heart failure specialist nurse | 16 | 48 |
| Other (not specified) | 5 | 15 |
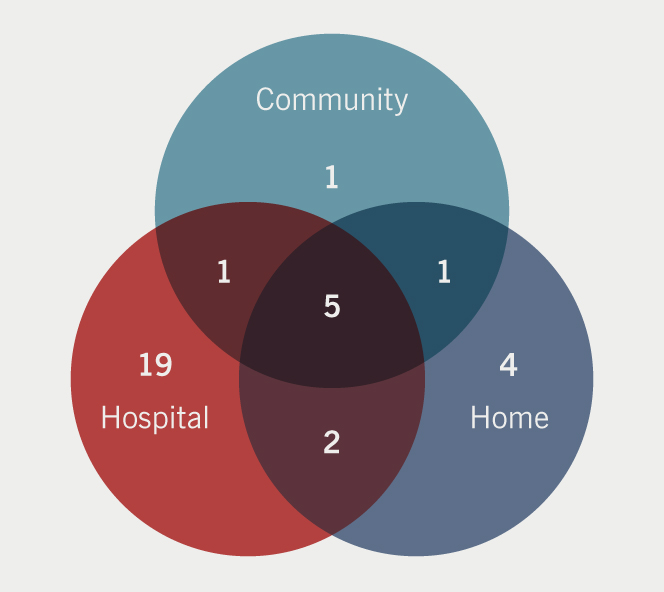
The most common site for OP treatment was in hospital settings (furosemide lounge/ambulatory care unit). A number of centres are able to provide parenteral diuretics at home or in the community as shown in figure 1.
The most common route for parental diuretics administration for OPM of ADHF was IV based, although some centres can deliver diuretics SC (20 IV only, seven IV and SC, four SC only, and two ‘don’t know’). Table 1 details which healthcare workers (HCW) are responsible for parenteral OP diuretics.
Ten (20%) of HF centres in the survey offered a clinical psychology service to HF patients, while 6% of centres were not sure whether they have this service.
Twenty-five sites expressed an interest to be in the steering group of the SAFE RCT, and 43 (86%) of the respondents would like to see the report of the survey.
The geographical location of the hospitals offering parenteral diuretics is available in supplementary table 2 (online).
Supplementary table 2. Hospital location by country within the UK.
| Country | Hospital * |
| England | Barnet Hospital, London Blackpool Victoria Hospital Bradford NHS foundation Trust Broomfield Hospital, Chelmsford Chelsea and Westminster Hospital, London Croydon Health Service NHS Trust Diana, Princess of Wales Hospital, Lincolnshire Doncaster Bassetlaw Teaching Hospitals NHS Foundation Trust Glenfield Hospital, Leicester Hillingdon Hospital, London Imperial College Healthcare NHS Trust, London James Cook University Hospital, Middlesbrough John Radcliffe Hospital, Oxford King’s College Hospital, London Lister Hospital, Hertfordshire London Northwest University Healthcare NHS Trust (Ealing, Northwick Mary’s) Medway Maritime Hospital, Kent Northwick Park Hospital, London Northwest University Healthcare NHS Trust Northern General Hospital, Sheffield Teaching Hospitals NHS Foundation Trust Northumbria Specialist Emergency Care Hospital Oxford University Hospitals NHS Foundation Trust Princess Royal Hospital, Telford Queen Alexandra Hospital, Portsmouth Royal Berkshire Hospital, Berkshire Royal Devon and Exeter NHS foundation Trust Royal Free Hospital, London Royal London, Whipps Cross, Newham General, Barts Hospital, London Royal University Hospitals, Bath Sandwell and City Hospital Birmingham Somerset NHS Foundation Trust Southmead Hospital, Bristol Southend Hospital/Basildon ECTC St George’s Hospital, London The Great Western Hospital, Swindon Torbay Hospital, Devon University Hospital of North Tees University Hospital Southampton West Middle University Hospital West Hertfordshire Hospitals NHS Trust Worcestershire Acute Hospitals NHS Trust Wycombe Hospital, Buckinghamshire Wythenshawe Hospital, Manchester |
| Northern Ireland | Ulster Hospital, Dundonald |
| Scotland | Ninewells Hospital, Dundee Raigmore Hospital NHS Highland University Hospital Monkland University Hospital Wishaw NHS Lanarkshire University Hospital Hairmyres |
| Wales | Princess of Wales Hospital, Bridgend University Hospital of Wales, Cardiff |
| * Two respondents did not disclose hospital name | |
Discussion
According to our 2021 survey, 9.5% of patients with ADHF were treated per year by IV diuretics as outpatients. There are 33 (65%) HF services that provide OPM for ADHF. Of the 17 services that do not currently offer OPM, 11 hope to develop this service. Only 10 (20%) HF services provide clinical psychology support to HF patients. Our survey shows that OPM for ADHF is becoming popular in the UK (65% of respondents indicated this strategy is available for their patients). This contrasted with 25% of HF services (only 14) providing OPM for ADHF in 2016.17
The response rate for our online survey across the UK (<60 responses) is comparable with a similar survey in 2016. In 2016, Mohee and Wong also used SurveyMonkey® (Palo Alto, Calif) for their online survey in 2016. The link was sent to 237 consultant cardiologists with an interest in HF in the UK identified from the Directory of Cardiology 2014. Again, fewer than 60 responded. Nevertheless, it is concerning that despite lack of RCT evidence demonstrating safety and effectiveness, there appears to be a rise in the proportion of centres offering OP-based AHF services. The observational studies suggest that this service may be safe for selected patients.13 However, section bias in observational studies makes it imperative that a multi-centre RCT is performed before results can be generalised. This may be the reason why there are no guidelines supporting the practice.
In the present survey, respondents estimated 25,135 patients per year (median 600 per site) are treated by inpatient care for ADHF; 2,631 patients per year (median 50 per site) receive OPM for ADHF. According to a NHFA summary report, about 74,696 patients were admitted to hospitals in England and Wales due to HF between April 2018 and March 2019. Thus, our sample included a substantial proportion of the total number of patients with AHF in the UK.
A recent systematic review,13 rightly calls for a RCT to establish effectiveness of an OPM strategy for management of AHF. In Mohee and Wong’s 2016 survey, 37.5% expressed interest to participate in a RCT to compare OP versus IP for the management of ADHF. There appears to be rising interest to address this important gap in our knowledge of the best way to deliver treatment for this very common medical problem, as 37 centres (73%) indicated interest to collaborate in a multi-centre RCT comparing the two treatment strategies in our 2021 survey. This may reflect the recognition among a large proportion of HF specialists that there is uncertainty over whether OPM is indeed safe and effective.
Our survey confirms there is uncertainty among the HF community about whether to develop this service. Of the 33% of centres that do not have this service, 11 hope to develop this service, while four do not know if they should develop this service. Two sites had no intention of developing this service. One might expect the true proportion of sites without this service, or which are uncertain about development of OPM, is substantially higher. Those sites may not be interested in completing such a survey. The planned large multi-centre trial will help to resolve the uncertainties HF services have regarding whether to develop OP-based IV diuretic treatment for AHF.
The rising interest of respondents in taking part in an RCT to compare OPM and standard IP care is encouraging. However, it should be noted that this may be because the 2021 survey was sent via the UK HF Research Investigator Network and UK CRNs.
The small pilot trial in Blackpool suggested that OPM is effective, safe and cost-effective, and is a strategy favoured both by patients and carers. Importantly, patients randomised to OPM appeared to enjoy an improvement of their mental well-being. OPM saves the NHS >£2,600 per patient.15 Nevertheless, a word of caution was expressed by the principal author of the pilot trial at the British Cardiovascular Society 2021.16 While patients randomised to OPM appeared to have increased levels of hope initially, by 60 days’ follow-up, their levels of hope dropped, possibly because there was a numerically (albeit not statistically significantly) higher number of readmissions by 60 days. Hopelessness was found to predict death in 2,428 men aged 42–60 years old.18 However, hope, defined as a positive psychology construct, comprises of state hope (which is one’s goal-directed thinking in any given moment and situation), and trait hope (that is a person’s disposition or general way of goal-directed thinking and, hence, more stable).19 Hope is associated with good clinical outcomes in patients with chronic health conditions.20,21 It is, therefore, important to address whole-patient care needs in patients with AHF.
Future directions
Thirty-five centres are interested to collaborate in a multi-centre observational study comparing OPM versus standard IP care for patients with acute/decompensated HF. Furthermore, 37 centres would like to collaborate in a multi-centre RCT comparing the two treatment strategies. An estimated 3,223 patients are eligible to take part in the RCT over two years (median 57 per site, IQR 34–100). The survey respondents estimated they could randomise 1,401 patients into a multi-centre RCT to compare IPM versus OPM for AHF within two years (median 30 per site, IQR 20–50).
In conclusion, OPM for HF with IV diuretics is becoming popular in the UK. Clinical psychology needs expansion to achieve our mission of providing excellent whole-person care for all patients with HF. A multi-centre RCT is needed to compare the safety and cost-effectiveness of OPM versus IP care for ADHF. Our survey suggests such a trial is feasible. It is clear there is a lot of interest among UK HF services to consider establishing OP services for the management of ADHF, and they recognise the need to resolve uncertainties about safety and effectiveness.
Key messages
- Of the sites that responded, it was estimated that nearly 10% of patients with acute decompensated heart failure (ADHF) are managed with ambulatory parenteral diuretics
- Before further rapid expansion of outpatient-based intravenous diuretic services in the UK, a multi-centre randomised-controlled trial is urgently needed to test the safety and cost-effectiveness of this innovative service. Our survey suggests such a trial is feasible
- It is clear there is a lot of interest among UK heart failure services to consider establishing outpatient services for the management of ADHF, and they recognise the need to resolve uncertainties about safety and effectiveness
Conflicts of interest
None declared.
Funding
None.
Acknowledgement
Mr Ben Hardwick, Liverpool Clinical Trials Centre, for helping us send the survey to Clinical Research Networks in England.
Study approval
According to guidance from the Health Research Authority, our study type will not be considered as research and as such would not require any approval.22
References
1. British Heart Foundation. Heart failure hospital admissions rise by a third in five years. 4 November 2019. Available at: https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2019/november/heart-failure-hospital-admissions-rise-by-a-third-in-five-years
2. National Institute For Cardiovascular Outcomes Research (NICOR). National Heart Failure Audit (NHFA): 2020 summary report (2018/19 data). London: Healthcare Quality Improvement Partnership (HQIP), 2020. Available from: https://www.nicor.org.uk/wp-content/uploads/2020/12/National-Heart-Failure-Audit-2020-FINAL.pdf
3. Sbolli M, Fiuzat M, Cani D et al. Depression and heart failure: the lonely comorbidity. Eur Heart J 2020;22:2007–17. https://doi.org/10.1002/ejhf.1865
4. British Heart Foundation. Heart statistics. Available at: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics [accessed 19 June 2021].
5. Desai AS, Stevenson LW. Rehospitalization for heart failure. Circulation 2012;126:501–06. https://doi.org/10.1161/CIRCULATIONAHA.112.125435
6. Afari ME, Aoun J, Khare S, Tsao L. Subcutaneous furosemide for the treatment of heart failure: a state-of-the art review. Heart Fail Rev 2019;24:309–13. https://doi.org/10.1007/s10741-018-9760-6
7. Ioannou A, Browne T, Jordan S et al. Diuretic lounge and the impact on hospital admissions for treatment of decompensated heart failure. QJM 2020;113:651–6. https://doi.org/10.1093/qjmed/hcaa114
8. Buckley LF, Seoane-Vazquez E, Cheng JWM et al. Comparison of ambulatory, high-dose, intravenous diuretic therapy to standard hospitalization and diuretic therapy for treatment of acute decompensated heart failure. Am J Cardiol 2016;118:1350–5. https://doi.org/10.1016/j.amjcard.2016.07.068
9. Ryder M, Murphy NF, McCaffrey D et al. Outpatient intravenous diuretic therapy: potential for marked reduction in hospitalisations for acute decompensated heart failure. Eur Heart J 2008;10:267–72. https://doi.org/10.1016/j.ejheart.2008.01.003
10. British Heart Foundation. Learning points for the successful introduction of Intravenous diuretics in the community. London: BHF, 2015. Available from: https://www.bhf.org.uk/informationsupport/publications/healthcare-and-innovations/learning-points-for-the-successful-introduction-of-intravenous-diuretics-in-the-community
11. British Heart Foundation. Treating heart failure patients in the community with intravenous diuretics. London: BHF, 2015. Available from: https://www.bhf.org.uk/informationsupport/publications/healthcare-and-innovations/intravenous-diuretics-in-the-community_sirivd1
12. Ahmed FZ, Taylor JK, John AV et al. Ambulatory intravenous furosemide for decompensated heart failure: safe, feasible, and effective. ESC Heart Failure 2021;8:3906–16. https://doi.org/10.1002/ehf2.13368
13. Wierda E, Dickhoff C, Handoko ML et al. Outpatient treatment of worsening heart failure with intravenous and subcutaneous diuretics: a systematic review of the literature. ESC Heart Fail 2020;7:892–902. https://doi.org/10.1002/ehf2.12677
14. Wong K, Latt NKZ, Debski M et al. Safety and effectiveness of outpatient based acute heart failure care: a randomised controlled feasibility trial. Heart 2020;106(suppl 2):A74–A75. https://doi.org/10.1136/heartjnl-2020-BCS.96
15. Wong K, Assaf O, Latt NKZ et al. Is outpatient based acute heart failure treatment cost-effective? An analysis based on a pilot prospective trial. Heart 2020;106(suppl 2):A73–A74. https://doi.org/10.1136/heartjnl-2020-BCS.95
16. Wong K, Hughes DA, Debski M et al. Does outpatient based IV diuretic treatment for acute heart failure give patients hope. Heart 2021;107(suppl 1):A116–A117. https://doi.org/10.1136/heartjnl-2021-BCS.149
17. Mohee K, Wong K. Are we ready for outpatient acute heart failure management (frusemide lounges and beyond)? A nationwide survey of UK acute heart failure practice. Heart 2016;102(suppl 6):A6-A7. https://doi.org/10.1136/heartjnl-2016-309890.9
18. Everson S, Goldberg D, Kaplan G et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Pyschosom Med 1996;58:113–21. https://doi.org/10.1097/00006842-199603000-00003
19. Snyder CR. Handbook of hope: theory, measures, and applications. San Diego, CA: Academic Press, 2000.
20. Billington E, Simpson J, Unwin J, Bray D, Giles D. Does hope predict adjustment to end-stage renal failure and consequent dialysis? Br J Health Psychol 2008;13:683–99. https://doi.org/10.1348/135910707X248959
21. Kortte KB, Stevenson JE, Hosey MM, Castillo R, Wegener ST. Hope predicts positive functional role outcomes in acute rehabilitation populations. Rehabil Psychol 2012;57:248–55. https://doi.org/10.1037/a0029004
22. Health Research Authority. What approvals and decisions do I need? https://www.hra.nhs.uk/approvals-amendments/what-approvals-do-i-need/