Implantable mechanical circulatory support systems have evolved dramatically over the last 50 years. The objective has been to replace or support the failing left ventricle with a device that pumps six litres of blood each minute, a massive 8,640 litres per day. Noisy cumbersome pulsatile devices have been replaced by smaller silent rotary blood pumps that are much more patient friendly. Nonetheless, the tethering to external components, together with the risks of power line infection, pump thrombosis and stroke, must be addressed before widespread acceptance. Infection predisposes to thromboembolism, so elimination of the percutaneous electric cable has the capacity to transform outcomes, reduce costs and improve quality of life.
Developed in the UK, the Calon miniVAD is powered by an innovative coplanar energy transfer system. As such, we consider it can achieve those ambitious objectives.
Introduction

Many of us have watched severe heart failure patients die miserably during haemorrhagic pulmonary oedema. The first for me was my 60-year-old grandfather when I was seven years old. Not something that was easily forgotten.
Months later, in 1955, I watched the first episode of ‘Your life in their hands’ from the Hammersmith Hospital. They talked of open heart surgery using something called cardiopulmonary bypass. It was then, in the backstreets of a northern steel town, that I decided to be a heart surgeon.
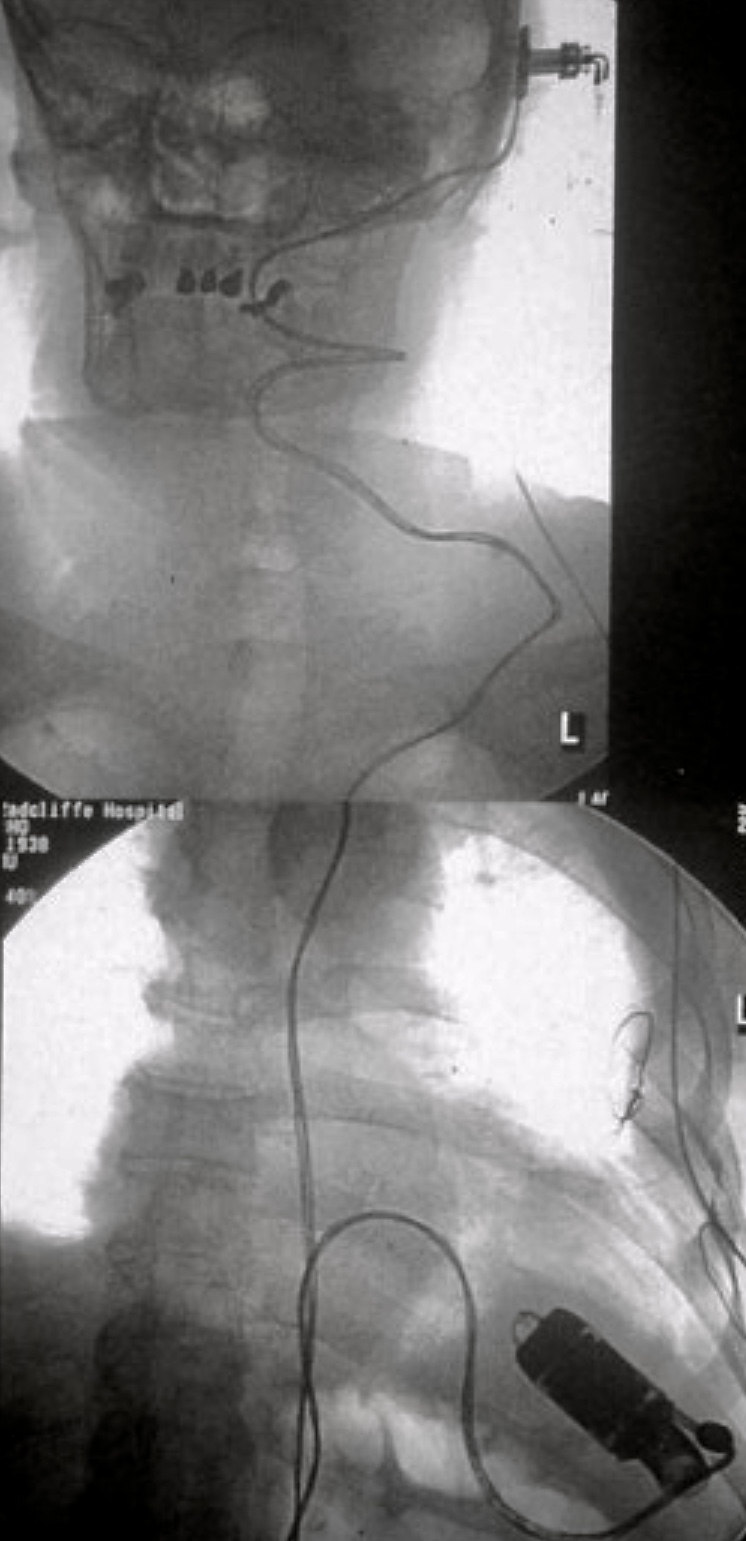
Fifty years later, when the BBC resurrected the series, I was filmed implanting a unique new artificial heart at the Royal Brompton Hospital. For that device, I introduced electrical energy into the patient through a titanium bolt screwed into the skull, just like Dr Frankenstein (figure 1). Why do such an outrageous thing? Because the percutaneous drivelines exiting the abdominal wall for other pumps frequently became infected and precipitated the patient’s death. By then I was well familiar with that scenario. Rigid fixation helped but did not eliminate the problem, nor was drilling into the skull something that most heart surgeons wished to do in the middle of a high-risk operation. And there were other issues associated with power. After two years of symptom-free life, that patient went out Christmas shopping without a spare battery. When the alarm sounded he tried to return home but didn’t make it. Pump off, life over.
Severe heart failure and the role of transplantation
There are reckoned to be between 100,000 and 150,000 stage D heart failure patients with systolic dysfunction under the age of 65 years in both North America and Europe. Most have renal impairment and pulmonary hypertension. Why discuss heart failure in an ageist manner? Because transplantation long since set that bar for eligibility, but should such restrictions really apply in the 21st century?
Stage D patients are unable to perform any physical activity without distressing breathlessness, and generally experience three urgent hospital admissions during their last six months of life. With escalating drug treatment and the need for pacemakers and implantable defibrillators, the costs of dying are considerable, and the end-of-life trajectory notoriously difficult to predict for any individual. In the control arm of the Randomised Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial (REMATCH), only 8% of patients were alive at two years and all remained severely incapacitated despite maximum medical therapy.1
I was a London medical student when Christiaan Barnard performed the first heart transplant in Cape Town. I recall contributing to a debate stating “no treatment that requires someone else to die first will ever prove mainstream”, and indeed others referred to the technique as “epidemiologically trivial”.2 As emotive as transplants proved to be, that remains the case. In Britain there are 40,000 heart failure deaths per year in this young age bracket and less than 200 donor hearts.
By the turn of the century, the limitations of cardiac transplantation were readily apparent. In highly selected candidates without renal impairment and with pulmonary vascular resistance <2.5 Wood units, waiting list mortality was between 8% to 10%.3 In an impressive analysis of 22,385 transplanted patients Kilic et al., in 2012, reported one-year mortality of 15%, while 58% had died within 10 years. For this group mean survival was 3.7 years.4 Of those exceeding the decade, median survival was 12.2 years. Clear predictors of longevity were age <55 years, white race, young donor age and short organ ischaemic time. Predictors of poor long-term survival were diabetes, obesity, hypertension and renal impairment, the precise characteristics of ischaemic cardiomyopathy patients. This suggests that a donor heart provides only limited benefit for those with the most common cause of heart failure.
Table 1. Cardiac transplant versus left ventricular assist device. Evidence-based preferred patient characteristics from a prognostic and economic standpoint3-6,8,9
| Transplant | LVAD | |
|---|---|---|
| Age, years | <55 | No limit |
| NYHA functional class | IV | III/IV |
| Urgency | LVAD or inotrope dependent | Elective |
| Peak VO2, ml/kg/min | <12 | <12 |
| LVEF, % | <25 | <25 |
| PVR, Wood units | <2.5 | <7.5 |
| CVP >20 mmHg | NA | CI |
| BMI >30 kg/m2 | CI | Acceptable |
| Renal impairment | CI | Acceptable |
| Diabetes | CI | Acceptable |
| Treated malignancy | CI | Acceptable |
| Vascular disease | CI | Acceptable |
| Key: BMI = body mass index; CI = contraindication; CVP = central venous pressure; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; NA = not applicable; NYHA = New York Heart Association; PVR = pulmonary vascular resistance | ||
Given the improvements in medical and non-transplant surgery in recent years, transplant survival advantage can only be discerned for United Network of Organ Sharing (UNOS) status 1 patients who are hospitalised on inotropes or mechanical circulatory support.5 Deng et al. showed that ambulatory (status 2) wait-listed patients, who did not receive an organ, had similar survival to those transplanted.5 Indeed, Shah et al. reported the one- and three-year survival of UNOS status 2 patients removed from a transplant waiting list to be 100% and 92%, respectively, with a third of them experiencing substantial improvement in native heart function when managed by an expert heart failure team.6 We also know that mechanical unloading of the failing left ventricle with an assist device promotes improvement in myocardial function.7
I should emphasise that I am very much pro-transplantation, not against it. As table 1 shows they are complementary, rather than competitive therapies, each employed for desperately sick patients. My only transplant in Oxford was for a child on a Berlin Heart who is alive with his own family 25 years later. But we have to be objective. Very few patients get that opportunity. Once the patient’s own heart is replaced, the clock starts ticking with outcomes limited by rejection episodes, opportunistic infection, cancer and allograft coronary artery disease.8 So why wouldn’t conservation of the native heart with support from an assist device prove preferable in the face of low complication rates.9 And why wait for terminal low cardiac output state with deteriorating renal state before intervening? Why accept borderline donor organs when a left ventricular assist device (LVAD) proves the safer option?
The difficulties in pumping blood
I was fortunate to have interface with mechanical circulatory support devices from an early stage of my career. Fortunate because they save those who are otherwise certain to die, and, while the NHS will not fund them, it insists upon publishing surgeons’ mortality rates. A curious paradox.
In 1981, I was training with John Kirklin at the University of Alabama. Kirklin was the great academic cardiac surgeon who succeeded in introducing the heart–lung machine after its inventor John Gibbon had abandoned its use in despair. But even at that stage there was one great problem with the technology. The interaction of blood, a delicate substance, with pumps and synthetic foreign surfaces caused life-threatening organ damage, generally referred to as the post-perfusion syndrome. I had a degree in biochemistry, so Kirklin charged me with investigating the molecular mechanisms of this process, which I characterised as a ‘whole body inflammatory response’ triggered by complement activation.10 Activated neutrophils trapped in the pulmonary circulation released protease enzymes and oxygen free radicals causing pulmonary oedema. Identifying nylon as the trigger, then removing it from the oxygenator, made a huge difference to the safety of cardiac surgery, and stimulated my longstanding interest in blood handling by mechanical devices. It was in Alabama that I first encountered the intra-aortic balloon pump, an interesting concept, but it couldn’t save patients in shock. That needs blood flow.
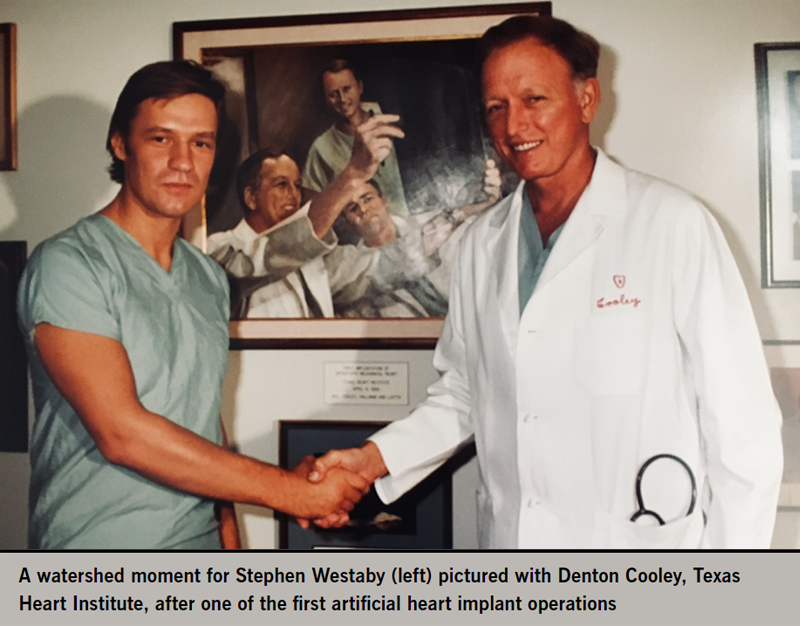
Then came the watershed moment for my whole career. It was 05.30 am on Friday 24 July 1981. When I arrived for the early morning intensive care round, there was a palpable buzz of excitement among the residents, so I enquired why? The previous evening at the Texas Heart Institute, Denton Cooley had implanted his, and the world’s, second total artificial heart (TAH), 14 years after the disastrous first. I was determined to see it, so that evening I took the ‘red eye’ flight to Houston.
Cooley treated the English interloper with kindness. We had something in common having both worked at the Brompton with Oswald Tubbs. That Saturday afternoon the patient was doing badly. Not only was the bulky pulsating device haemolysing the blood, it was also distorting the pulmonary veins causing pulmonary oedema. Another new technique, veno-venous extracorporeal membrane oxygenation (ECMO) was being used to treat severe hypoxia while they desperately searched for a donor organ.11 I watched the transplant rescue procedure in the early hours of 25 July, but the patient died a week later. Nonetheless, within weeks new artificial heart programmes had emerged in the US, Russia, China, Japan, Argentina and several European countries.
Coincident with this second disappointing effort, the artificial heart engineer, Robert Jarvik of Utah, wrote in Scientific American “If the artificial heart is ever to achieve its objectives it must be more than a pump, more than functional, reliable and dependable. It must be forgettable”. By this he meant that implanted blood pumps should not impact negatively upon the patient’s quality of life.
When I returned to London in 1982, my mind was buzzing with balloon pumps, ECMO and artificial hearts. Far-fetched though they seemed to my colleagues, the objectives to relieve suffering and prolong lives were perfectly sound. That is what heart surgeons and health organisations are meant to do, so the sooner we had them in the UK, the better.
That December, after more than US$160 million in Federal funding for the technology, the first intended permanent implant of a TAH, the Jarvik 7, took place at the University of Utah.12 Was the device as dependable and patient friendly as the engineer had wished? Hardly. The 61-year-old dentist, Barney Clarke, was tethered, for what remained of his life, to a noisy external air compressor. Next, the sternum could not be closed over the bulky mechanical ventricles. Then, on post-operative day 13, the Bjork Shirley mitral prosthesis suffered a strut fracture and had to be replaced urgently. Poor Clarke lurched from one catastrophe to another, eventually dying from multi-organ failure 112 days after the implant.
As for the recent pig heart transplant patient in the US, Clarke had expressed his eagerness to be given the chance of life.12 He told his cardiologist that he had given up his favourite pastime of fishing “because I cannot stand to see the fish gasping for breath on the dock”. When shown the calves with artificial hearts in the laboratory, he said “these animals cannot speak but I believe they feel much better than I do”. My grandfather would have agreed with him.
Evolving circulatory support technology
In 1986, I was taken on to develop cardiothoracic surgery in Oxford, but continued to collaborate with Houston. Cooley went on to perform more heart transplants than anyone else, and was well aware of their limitations. Supported by his colleague O H Frazier, the laboratories at the Texas Heart Institute became the testing ground for many new blood pumps designed for both temporary and permanent circulatory support. And because of the restrictions imposed on human implants by the Food and Drug Administration (FDA), I persuaded them to consider Oxford as an alternative clinical centre. Cambridge were exploring xenotransplantation, so it became a bit of a boat race.
With time, it became apparent that biventricular cardiac replacement was seldom necessary. The failing left ventricle pumps against much greater afterload, and support for the systemic circulation was usually sufficient to reverse heart failure and provide symptomatic relief. Pulsatile volume displacement ventricular assist devices then emerged, and were widely used for bridge to transplant. These cumbersome pumps had a mechanical durability up to two years, with relatively low thromboembolic rates, but remained subject to debilitating power line infections.13
The portable electric ThermoCardiosystems LVAD was first implanted by Frazier at the Texas Heart Institute in 1991, and I went to watch the procedure. It was used for a young, end-stage dilated cardiomyopathy patient whose heart failure resolved in days. Under the terms of the investigational agreement, the man was restricted to the hospital grounds while waiting for a donor heart but employed in computer work to keep him occupied. Depressed by his prolonged confinement, he stopped taking warfarin and suffered a catastrophic stroke. But when the LVAD was switched off following 18 months of support, his own heart continued to produce normal cardiac output. Native left-ventricular function was virtually normal. This opened up the prospect of a mechanical bridge to recovery for some cardiomyopathy patients, and persuaded Frazier that the electric LVAD could be employed as a permanent solution for non-transplant eligible patients.14 So Houston came to Oxford.
Artificial hearts were somewhat of a departure for the NHS, so I turned to the Brompton for some heavyweight heart failure cardiology. Philip Poole-Wilson had many debilitated patients who were desperate for symptomatic relief. In order to receive regulatory approval, we had to select non-transplant eligible candidates with raised pulmonary vascular resistance who were expected to die within six weeks. This proved a tall order from the anaesthesia and intensive care perspective, but fortunately the two cases went well. I could only afford two because all costs had to be covered by my research funds. Breathlessness and fatigue resolved rapidly but, after several months, driveline infection ensued, and we lost them from sepsis.
In the meantime, we achieved a series of ‘bridge to recovery’ successes using new extracorporeal support circuits in patients with myocardial infarction, then viral and phaeochromocytoma myocarditis. If a high-risk patient could not be weaned from cardiopulmonary bypass, we transferred them to a temporary external system for a matter of days until they recovered. And we used ECMO for swine flu. The objective was to take on everyone who might benefit, whatever the risk, and not to lose anyone.
The bulky pulsatile artificial ventricles, intended to replicate human physiology, were never patient friendly, nor sufficiently mechanically reliable for long-term use. The emergence of compact high-speed implantable rotary blood pumps occurred through serendipity. Texas engineer, David Saucier, designed the huge turbo pumps that feed liquid hydrogen propellant into the space shuttles’ main engines. After a myocardial infarction in 1984, Saucier needed a cardiac transplant by the circulatory support pioneer, Michael DeBakey, at Baylor College of Medicine. While recovering, DeBakey asked him to design a miniature high-speed rotary blood pump, fully anticipating that it would destroy red cells like an egg whisk. It didn’t.
In those days, both physiologists and surgeons believed that pulse pressure was fundamental to mammalian physiology. Many even believed that the limited periods of cardiopulmonary bypass during cardiac surgery should be made pulsatile. They were wrong. With rotary LVADs some pulse pressure transmitted through the device is desirable to stop the formation of arterio-venous malformations in the gut, but it is flow that is important. We demonstrated that conclusively in the laboratory in Oxford.
The Baylor collaboration produced the MicroMed DeBakey LVAD, a silent implantable pump producing continuous systemic flow with limited blood damage.15 Saucier survived for 12 years with his donor heart and other turbine pumps followed, notably the Jarvik 2000.16 By then, Jarvik had his own company in a Manhattan skyscraper. I met him by chance at the 1996 US Society for Thoracic Surgery meeting in San Antonio. He produced a thumb-sized titanium cylinder with its torpedo shaped rotor from his briefcase, plugged it in and placed it in a bowl of water. Whoosh. I said “that’s a great pump for water but it will haemolyse blood”. Opportunistically, I followed with “but I would like to test it in my lab in Oxford”. I didn’t have a laboratory, but after diverting to the Texas Heart Institute to see the device in calves, I went home and established one.
In spring 2000, Frazier began a bridge-to-transplant trial with the Jarvik pump, and I scrubbed in with Cooley and Frazier for the first case. Of course, there was a percutaneous electric cable emerging from the abdominal wall, and while more flexible than the pulsatile LVAD drivelines, it was still subject to infection. In Oxford, we developed an innovative alternative to the abdominal exit site. Skull pedestal power delivery emerged in an effort to combat disruptive movement between cable and skin for our ‘mechanical alternative to transplant’ initiative (figure 1).17
With the backup of Poole-Wilson and Oxford’s Adrian Banning, we performed the world’s first permanent implant of a rotary blood pump on a desperately sick patient in July 2000. A pulseless Peter Houghton, who was twice denied transplantation, experienced symptom-free life without thromboembolism for almost eight years.18,19 That was by far the longest survival with any type of artificial heart, and he eventually succumbed to failure of his single diseased kidney. Did the skull pedestal remain infection free? Sadly not. Moreover, several other of my patients died from power cable complications. But at least we won the boat race. Xenotransplant survival in the animal laboratory was still measured in days.
British and best
When Houghton reached the landmark five-year milestone, we were given a reception at number 10 Downing Street. At the gathering, our animated patient lamented the fact that the NHS would not fund LVAD technology for others, and suggested that we should establish our own development programme. A Welsh contact of Peter’s, the physicist and entrepreneur Marc Clement, who began laser hair removal, was present that evening and we agreed to pursue the suggestion. We brought together the skills of motor engineers and experts in computational fluid dynamics under the umbrella of Calon CardioTechnology. In time, we also engaged a chief executive officer, Stuart McChonchie, who had previously taken the HeartWare and Jarvik 2000 LVADs through the stages of investigational and regulatory approval.
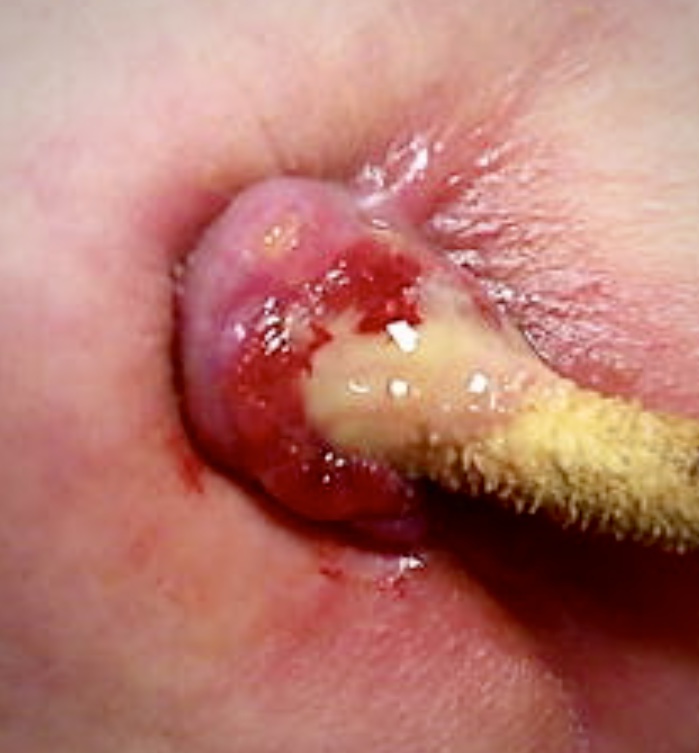
Why develop another LVAD in the UK? Quite simply because these devices needed to evolve further in terms of size, safety and affordability. I was also a paediatric cardiac surgeon, and wanted to produce a device suitable for children. That had to be a better pump. It was obvious that blood handling was the key to minimising LVAD-related complications, and it was equally apparent that driveline problems would remain the Achilles’ heel of pumps that were kind to blood.20 As many as 40% of patients suffer percutaneous power cable infection within two years (figure 2). Inflammation promotes thrombotic complications by initiating clotting, decreasing the activity of natural anticoagulant mechanisms and impairing the fibrinolytic system, and thrombus results in thromboembolism. Once a pump is infected it has to come out.
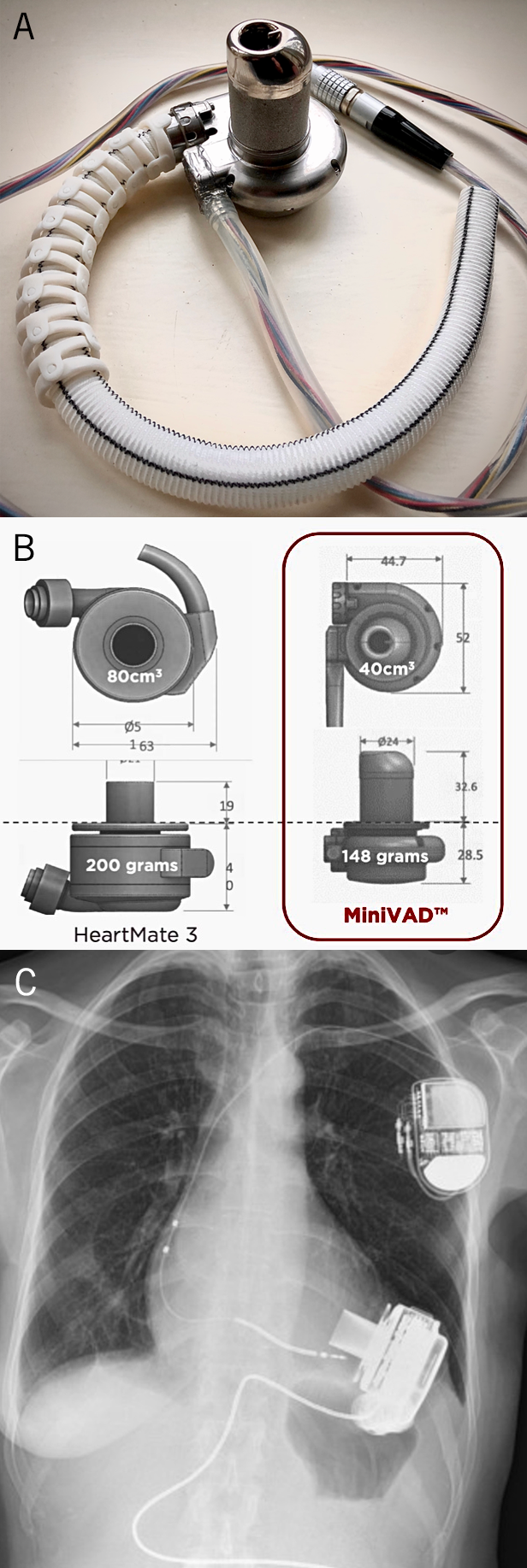
B. Comparative sizes (in millimetres) of the Calon MiniVAD and HeartMate 3 device. C. X-Ray showing the HeartMate 3 device implanted
At Calon we produced a rotary blood pump (figure 3A) that is substantially smaller than the HeartMate 3. Like its predecessors, our device went through several design iterations, subject to detailed testing, until it handled blood significantly better than competitors in a sophisticated mock circulatory. For years we have monitored haemocompatability by measuring full blood counts, plasma free haemoglobin, the normalised index of haemolysis, platelet activation, Von Willebrand factor and leukocyte death over time. During those tests, the Calon pump consistently produced the lowest plasma free haemoglobin and four times less haemolysis than the significantly larger market leading HeartMate 3 device (figure 3B and 3C). The other main competitor, the HeartWare LVAD, has now been withdrawn from clinical use by Medtronic because of mechanical unreliability and a worrying incidence of thromboembolic events.21
The implanted components of all rotary blood pumps are silent, and generally cannot be felt within the body. However, the percutaneous cable, together with perpetual tethering to external batteries and controller, detract from quality of life, increase costs and contribute to mortality in one-third of LVAD recipients. Once driveline infection is established, continuous contact with the hospital is required for antibiotics and surgical revision of the exit site. So the ideal solution is to eliminate the power cable altogether. Potential solutions have been worked on for years, but no implantable energy source exists that would last the duration of survival, or have the capacity to recharge without piercing the skin. Nuclear batteries have been considered, and indeed tested, but the potential dangers of radioactivity together with costs have prevented their introduction into clinical practice. So what are the possibilities given that uninterrupted power is required to pump that 8,640 litres of blood per day.
Transcutaneous energy transmission
As early as 1961, Schuder et al. described an inductive coupling arrangement of two pancake-shaped coils that could transfer electromagnetic energy across a closed chest wall.22 This work led to transcutaneous energy transfer systems (TETS), whereby external direct current power from a control module is first converted into a higher frequency alternating current by a power inverter. A primary transmitter coil then transfers the power by inductive coupling across the skin to a subcutaneously implanted receiver coil, which must remain closely opposed. Finally, the internal alternating current is switched back to direct current to supply an internal motor. When in close proximity, TETS coils generate sufficient power to supply an LVAD with up to 72% efficiency.
The first commercial devices to be implanted with TETS were the AbioCor TAH and the LionHeart LVAD.23,24 Neither device proved successful in the clinical arena, but the experience clearly demonstrated the value of eliminating driveline infection and consequent sepsis. Energy transfer problems were experienced frequently through malalignment of the sensitive coils. Though TETS is still being explored, the transmission range and coil alignment issues remain a critical limitation. What’s more, this mode of energy transmission, in itself, consumes 20% of the power input, substantially less efficient than a percutaneous driveline. Should the distance between the external and subcutaneous coils increase, power transmission falls off markedly, and a power boost can burn the skin. As a result, unholstered use with TETS was limited to less than 30 minutes.
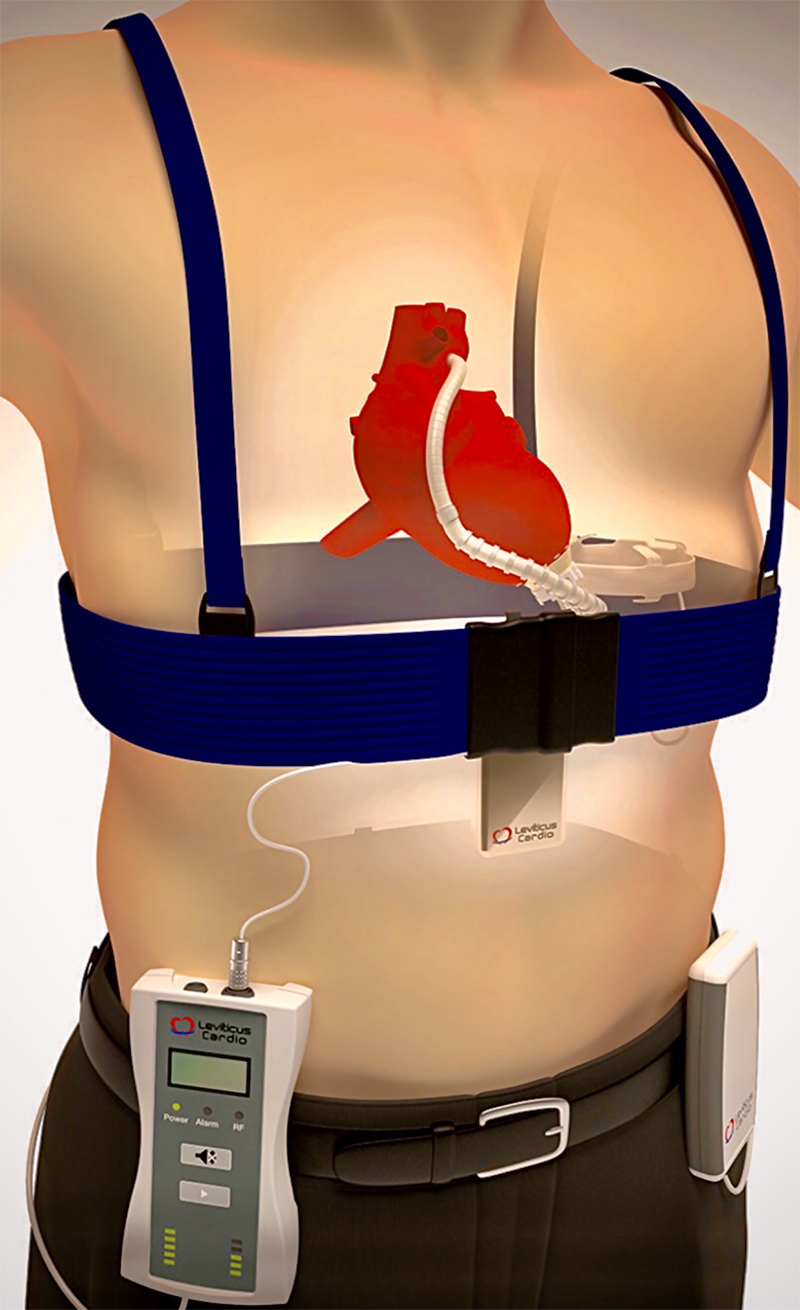
In contrast, we power the Calon MiniVAD with a unique coplanar energy transfer (CET) system, developed by the Leviticus Cardio Company (figure 4). This innovative system is based upon two rings, utilising coil-within-coil topology, to energise an internal battery that can provide prolonged tether-free LVAD powering.25 The external components worn to charge the implantables include a power transmission belt coupled to a portable controller battery and a wrist watch monitor. Within the patient are an integrated controller and battery coupled with the intrathoracic coil ring implanted around the base of the right lung. For energy harvesting while not wearing the externals, both pump and patient parameters are continuously recorded by the wrist monitor and alarm system. When power reserves fall the watch buzzer alarms, while there is still one hour of normal pump operation left. After that hour, a high-alert alarm will follow.
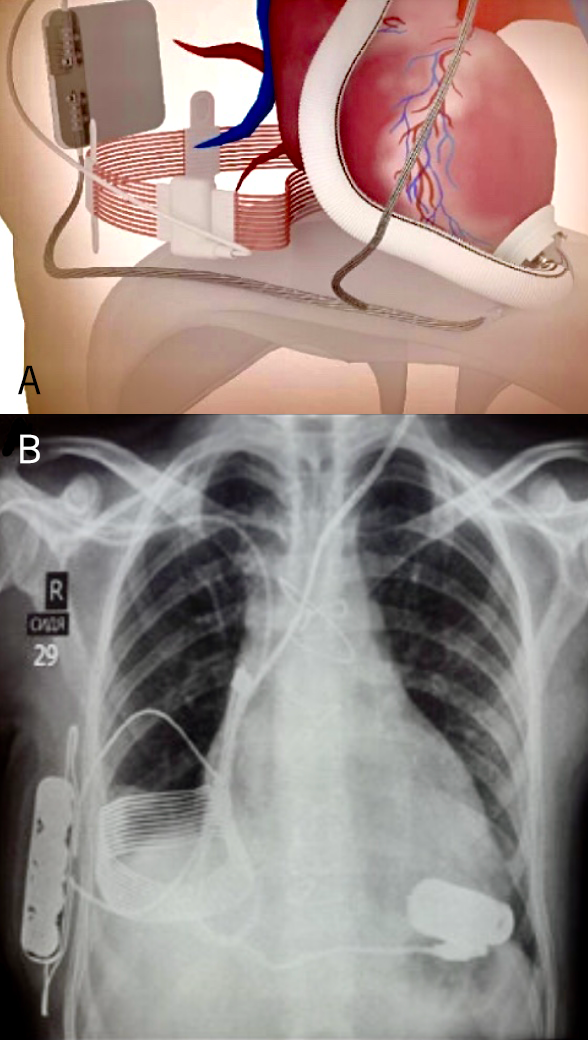
A. Diagram of the system.
B. Plain chest X-ray of the patient showing the implanted components. All data regarding power consumption and pump speed are relayed to the external wrist watch
After extensive laboratory testing, the CET system was implanted by Paya and colleagues into two bridge-to-transplant patients with the Jarvik 2000 Heart using an incorporated skull pedestal power line as backup (figure 5).25 It was not required. Without direct skin contact or heating, the CET supplied up to 30 Watts of power at 75% efficiency. Our pump requires less than 5 Watts. CET had low sensitivity to system displacement, movement or metals in proximity, and consistently provided more than eight hours of completely untethered activity. That is eight hours without any externals to impact upon the patient’s activities. Since then, we have validated the Calon MiniVAD with CET and incorporated the Leviticus Cardio Company into Calon CardioTechnology.
The last lap
I have argued that advanced heart failure symptoms are so continuously distressing that it is unreasonable, indeed unethical, to withhold any treatment with proven benefit. Patients with rhythm problems receive pacemakers, patients with crippling breathlessness should receive pumps.
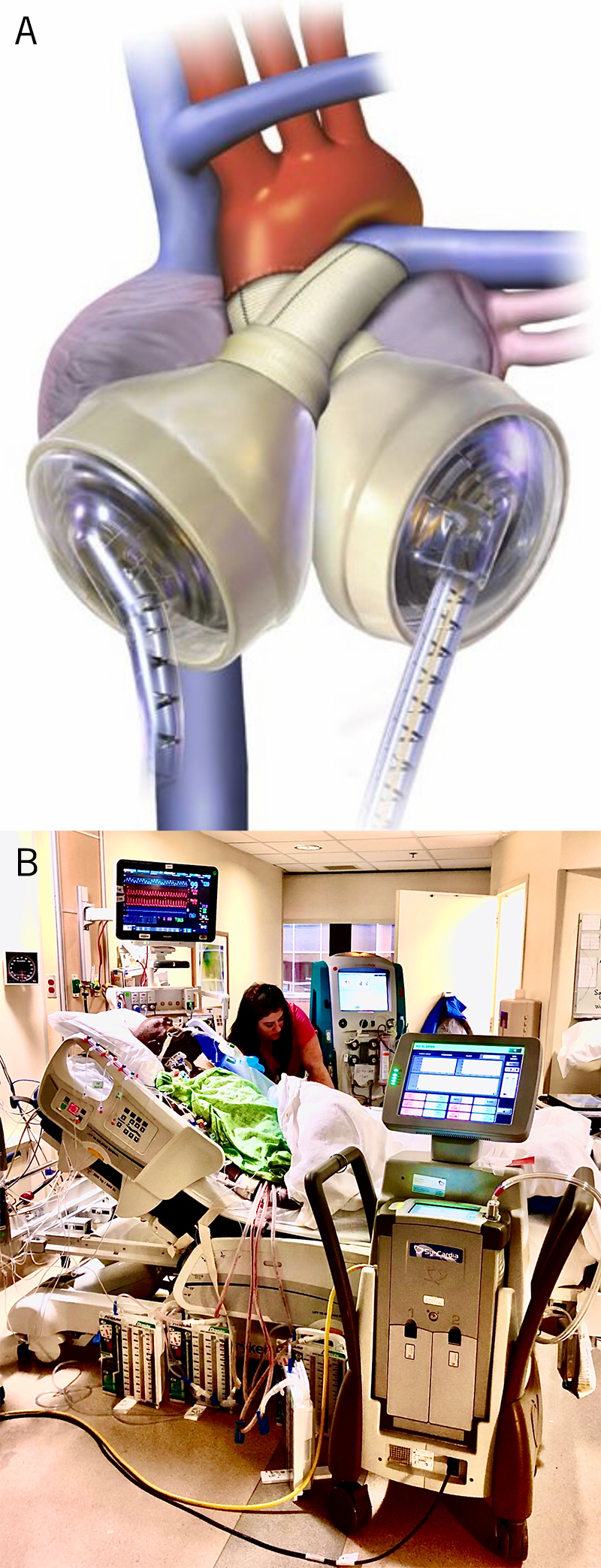
Is the right ventricle ever a problem in LVAD patients? It certainly can be. Two per cent of patients who have raised pulmonary vascular resistance require temporary right-sided support within two weeks of the implant, but after three months, problems with right ventricular failure are rare. However, stroke and gastrointestinal bleeding rates are higher when the right ventricle is struggling and the glomerular filtration rate falls. Bear in mind that transplantation is off limits for all patients with pulmonary hypertension, unless pulmonary vascular resistance falls with LVAD use over time.27,28 In the meantime, total artificial hearts are still used for bridge to transplantation in patients with severe right heart failure or cardiac tumours where the whole heart must be removed (figure 6).
It is more than 20 years since my operation on Peter Houghton. That gave an end-stage patient many years of good quality life, allowing him to travel to the US and Europe to demonstrate the efficacy of rotary blood pump technology. Subsequently, many LVAD patients have exceeded 10 years’ survival with few adverse events. Despite that, the NHS still does not recognise the LVAD as an alternative to transplantation.
Will pig hearts ever prove acceptable as an ‘off-the-shelf’ commodity, as the media suggests? The great transplant pioneer, Norman Shumway, used to say “xenotransplants are something for the future and always will be”. And notably his cardiac surgeon daughter, Sarah, is a VAD enthusiast.26 Whether those impressive genetic manipulations can provide a colony of pigs for slaughter on demand is one thing. The need for lifelong immunosuppression with its frequent complications is another. That said, the more methods we have to relieve the ravages of severe heart failure, the better.
Conclusion
We now suggest that an electively implanted tether-free LVAD will emerge as a better option than a donor heart, let alone one from a pig.9 As a manufactured product, the procedure has widespread applicability for all age groups without ethical, cultural or religious objections. What is more, LVADs are used to treat sicker individuals (table 1), and offloading the failing left ventricle helps to improve innate cardiac function. Elimination of driveline infection should, in turn, reduce the propensity for thromboembolism.29,30
We hope to introduce the Calon MiniVAD into clinical practice by the end of 2022.
Key messages
- For the past decade, electively implanted rotary blood pumps have provided symptomatic relief and a survival equivalent to cardiac transplantation
- Given the shortage of donor organs, and the complications of immunosuppression, set against substantial improvements in LVAD technology, mechanical circulatory support is emerging as the ‘gold-standard’ treatment for severe heart failure, without the limits of age or comorbidity
- It is inhumane and, arguably, unethical to deny symptomatic relief on the grounds of cost. All front-line cardiac centres should be equipped with ventricular assist devices, both temporary and permanent, just as they have pacemakers and implantable defibrillators
Conflicts of interest
As stated in the text, SW is founder and director of Calon CardioTechnology. After 30 years as senior cardiac surgeon in Oxford he retired from the NHS in 2017. He is author of the Sunday Times best sellers, Fragile Lives and The Knife’s Edge.
Funding
None.
Patient consent
Details of all patients described are in the public domain.
Editors’ note
Please see the editorial on this article by Professor Jignesh Patel which can be found here: https://doi.org/10.5837/bjc.2022.022.
References
1. Rose EA, Gelijns AC, Moskowitz AJ et al. Long term mechanical left ventricular assistance for end stage heart failure. N Engl J Med 2002;345:1435–43. https://doi.org/10.1056/NEJMoa012175
2. Redfield MM. Heart failure – an epidemic of uncertain proportions. N Engl J Med 2002;347:1442–4. https://doi.org/10.1056/NEJMe020115
3. Westaby S. Cardiac transplant or rotary blood pump. Contemporary evidence. J Thorac Cardiovasc Surg 2013;145:24–31. https://doi.org/10.1016/j.jtcvs.2012.08.048
4. Kilic A, Weiss ES, George TJ et al. What predicts long term survival after heart transplantation? An analysis of 9400 ten year survivors. Ann Thorac Surg 2012;93:699–704. https://doi.org/10.1016/j.athoracsur.2011.09.037
5. Deng MC, DeMeester JMJ, Smith JM et al. The effect of receiving a heart transplant: analysis of a national cohort entered into a waiting list stratified by heart failure severity. BMJ 2000;321:540–5. https://doi.org/10.1136/bmj.321.7260.540
6. Shah NR, Rogers JD, Ewald GA et al. Survival of patients removed from the heart transplant waiting list. J Thorac Cardiovasc Surg 2004;127:1481–5. https://doi.org/10.1016/j.jtcvs.2003.12.024
7. Westaby S, Coats AJ. Mechanical bridge to myocardial recovery. Eur Heart J 1998;19:541–7. https://doi.org/10.1053/euhj.1997.0985
8. Potena L, Zuckermann A, Barberini F et al. Complications of cardiac transplantation. Curr Cardiol Rep 2018;20:73. https://doi.org/10.1007/s11886-018-1018-3
9. Westaby S, Deng M. Continuous flow pumps: the new gold standard for advanced heart failure. Eur J Cardiothorac Surg 2013;44:4–8. https://doi.org/10.1093/ejcts/ezt248
10. Kirklin J, Westaby S, Blackstone EH. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983;86:845–57. https://doi.org/10.1016/S0022-5223(19)39061-0
11. Bartlett RH, Gazzaniga AB, Fong SW, Burns NE. Prolonged extracorporeal cardiopulmonary support in man. J Thorac Cardiovasc Surg 1974;68:918–32. https://doi.org/10.1016/S0022-5223(19)39687-4
12. Westaby S. Mechanical circulatory support. In: Landmarks in Cardiac Surgery. Oxford: Isis Medical Media, 1997; pp. 297–306.
13. McCarthy PM, Schmitt SK, Vargo RL et al. Implantable LVAD infections: implications for permanent use of the device. Ann Thorac Surg 1996;61:396–8. https://doi.org/10.1016/0003-4975(95)00990-6
14. Frazier OH, Benedict CR, Radovancevic B et al. Improved left ventricular function after chronic left ventricular unloading. Ann Thorac Surg 1996;62:675–82. https://doi.org/10.1016/S0003-4975(96)00437-7
15. Goldstein DJ. Worldwide experience with the MicroMed DeBakey ventricular assist device as bridge to transplant. Circulation 2003;108(suppl 2):272–7. https://doi.org/10.1161/01.cir.0000087387.02218.7e
16. Westaby S, Banning A, Jarvik R et al. First permanent implant of the Jarvik 2000 Heart. Lancet 2000;356:900–03. https://doi.org/10.1016/S0140-6736(00)02680-5
17. Westaby S, Jarvik R, Freeland A et al. Post auricular percutaneous power delivery for permanent mechanical circulatory support. J Thorac Cardiovasc Surg 2002;123:977–83. https://doi.org/10.1067/mtc.2002.121045
18. Saito S, Westaby S, Piggot D et al. End organ function during chronic non pulsatile circulation. Ann Thorac Surg 2002;74:1080–5. https://doi.org/10.1016/S0003-4975(02)03846-8
19. Westaby S, Banning A, Neil D et al. Optimism derived from 7.5 years of continuous flow circulatory support. J Thorac Cardiovasc Surg 2010;139:e45–e47. https://doi.org/10.1016/j.jtcvs.2008.05.072
20. Goldstein DJ, Naftel D, Holman W et al. Continuous flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 2012;31:1151–7. https://doi.org/10.1016/j.healun.2012.05.004
21. Kuehn BM. FDA: stop using Medtronic’s HeartWare ventricular assist device. JAMA 2021;326:215. https://doi.org/10.1001/jama.2021.11386
22. Schuder JC, Stephenson HE, Townsend JF. Energy transfer in a closed chest by means of stationary coupling coil and portable high power oscillator. Trans Am Soc Artif Intern Organs 1961;7:327–31.
23. Dowling RD, Gray LA, Etoch SW. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg 2004;127:131–41. https://doi.org/10.1016/j.jtcvs.2003.07.023
24. Mehta SM, Pae WE, Rosenberg G et al. The LionHeart LVD-2000: a completely implanted left ventricular assist device for chronic circulatory support. Ann Thorac Surg 2001;71(suppl):S156–S161. https://doi.org/10.1016/S0003-4975(00)02641-2
25. Paya Y, Maly J, Bekbossynova M et al. First human use of wireless coplanar energy transfer coupled with a continuous flow left ventricular assist device. J Heart Lung Transplant 2019;38:339–43. https://doi.org/10.1016/j.healun.2019.01.1316
26. Shumway SJ. Heart transplantation versus long term mechanical assist devices: clinical equipoise? Eur J Cardiothoracic Surg 2013;44:195–7. https://doi.org/10.1093/ejcts/ezt210
27. Stevenson LW, Hoffman JRH, Menachem JN. The other ventricle with left ventricular assist devices. J Am Coll Cardiol 2021;78: 2309–11. https://doi.org/10.1016/j.jacc.2021.09.1364
28. Lampert BC, Teuberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123–30. https://doi.org/10.1016/j.healun.2015.06.015
29. Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005;131:417–30. https://doi.org/10.1111/j.1365-2141.2005.05753.x
30. Davis PR, Miller-Dorey S, Jenne CN. Platelets and coagulation in infection. Clin Transl Immunology 2016;5:e89. https://doi.org/10.1038/cti.2016.39
