There have been several key developments in heart failure (HF) management since the 2016 European Society of Cardiology (ESC) HF guidelines were published. The updated 2021 ESC HF Guidelines reflect the modern management of the patient with HF, with an emphasis on evidence-based, individualised care. This overview aims to provide clinicians (whether specialist or non-specialist) with a summary of the major update in drug therapy for HF according to the recent 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF.
Introduction

Heart failure (HF) is a common condition and the majority of patients have multiple co-morbidities. It is therefore essential that all healthcare professionals (HCPs) are familiar with the contemporary management of these patients. Whilst HF specialists are integral to the delivery of optimal patient care, it is important to ensure that therapies are optimised at every opportunity and enable the best care for patients in the context of acute or chronic non-cardiovascular illness. Current practice is often suboptimal; for example, in the latest national HF audit (England and Wales), the number of patients leaving hospital on three disease-modifying drugs was 56% for those under the care of cardiology and 40% for those treated on general medical wards.1
This article will provide a practical synopsis of the recent 2021 ESC HF guidelines for cardiologists and non-cardiologists alike, including key evidence underpinning the recommendations.2
Pharmacologic therapies for HFrEF
The sequential initiation of medication with prognostic benefit has historically underpinned the treatment for patients with a diagnosis of HF with reduced ejection fraction (HFrEF). Such medications are typically considered before other therapies, including devices, with the goal of reducing the risk of death, hospitalisation for HF and improving symptoms. Until recently, there were three main groupings of drugs used:
- Angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB)
- Beta blockers
- Mineralocorticoid receptor antagonists (MRAs).
These drugs antagonise maladaptive neurohumoral activation, thought to be central to the pathophysiology of HF. As such, they are considered disease-modifying drugs. Large-scale trials over the last 30+ years have confirmed efficacy at reducing mortality and HF hospitalisations, often accompanied by improving symptoms (see table 1).3–16 There is a fundamental need to appreciate this, since in clinical practice it is common to see these drugs stopped or down-titrated due to the misconception that their primary use is to lower blood pressure or for diuretic effect. Such misunderstanding can lead to the inappropriate cessation of life prolonging therapy.
Table 1. Randomised trials studying the group of patients with a left ventricular ejection fraction (LVEF) ≤40% (except in the DAPA CKD study). The table shows A: trials with earlier disease-modifying drugs and B: those with more recent drug classes incorporated into the 2021 ESC guidelines
| Class of drug | Trial | Number of participants | Primary outcome (hazard ratio, p value) | Secondary outcome(s) (hazard ratio, p value) | |||
|---|---|---|---|---|---|---|---|
| A. Earlier disease-modifying drugs | |||||||
| ACE inhibitor/ARB | CONSENSUS (1987)3 Enalapril vs. placebo |
253 | All-cause mortality – 40% reduction at six months compared with placebo | ||||
| SOLVD-treatment (1991)4 Enalapril vs. placebo |
2,569 | All-cause mortality reduced with enalapril (0.84, p=0.004) | All-cause mortality/hospitalisation for HF (0.74, p<0.0001) | ||||
| ATLAS (1999)5 High- vs. low-dose lisinopril |
3,164 | All-cause mortality (0.92, p=0.13, non-significant reduction) | CV death (0.90, p=0.07, non-significant). All-cause mortality/CV hospitalisation CV death/CV hospitalisation (0.85, p<0.001) |
||||
| CHARM-added (2003)6 Candesartan and ACE inhibitor vs. placebo and ACE inhibitor |
2,548 | CV death or hospitalisation for HF (0.85, p=0.01) | |||||
| CHARM-alternative (2003)7 Candesartan vs. placebo (no ACE inhibitor) |
2,028 | CV death or hospitalisation for HF (0.77, p<0.001) | |||||
| Beta blocker | CIBIS-II (1999)8 Bisoprolol vs. placebo |
2,647 | All-cause mortality (0.66, p<0.001) | Reduction in sudden death (0.56, p=0.0011) | |||
| COPERNICUS (2001)9 Carvedilol vs. placebo |
2,289 | All-cause mortality (0.65, p<0.001) | All-cause mortality/hospitalisation (0.76, p<0.001) | ||||
| SENIORS (2005)10 Nebivolol vs. placebo in patients >70 years |
2,128 | All-cause mortality or CV hospitalisation (0.86, p=0.04) | |||||
| Mineralocorticoid receptor blocker | RALES (1999)11 Spironolactone vs. placebo |
822 | All-cause mortality (0.70, p<0.001) | ||||
| EMPHASIS HF (2011)12 Eplerenone vs. placebo |
2,737 | CV death or hospitalisation for HF (0.63, p<0.001) | |||||
| B. More recent drug classes | |||||||
| ARNI | PARADIGM HF (2014)13 Enalapril vs. sacubitril/ valsartan |
8,442 | CV death or hospitalisation for HF (0.80, p<0.001) | ||||
| SGLT2 inhibitors | DAPA HF (2019)14 Dapagliflozin vs. placebo |
4,744 | Worsening HF or CV death, with or without diabetes (0.74, p<0.001) | ||||
| DAPA CKD (2020)15 | 4,094 | ≥50% ↓ in eGFR or ESRD or death from renal/CV cause (0.61, p<0.001) | ≥50% ↓ in eGFR or ESRD or renal death (0.56, p<0.001). HF hospitalisations or CV death (0.71, p=0.009) Death any cause (0.69, p=0.004) |
||||
| EMPEROR-Reduced (2020)16 Empagliflozin vs. placebo |
3,730 | Worsening HF or CV death, with or without diabetes (0.75, p<0.001) | Hospitalisation for HF (0.70, p<0.001). Composite renal outcome (0.50) |
||||
| Key: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; CV = cardiovascular; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; ESRD = end stage renal disease; HF = heart failure | |||||||
The 2016 ESC guidelines incorporated the use of sacubitril/valsartan, an angiotensin receptor-neprilysin inhibitor (ARNI) as an alternative to ACE inhibitors or ARBs following the PARADIGM (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, which demonstrated a significantly reduced risk of all-cause mortality, death from cardiovascular (CV) causes and hospitalisation for HF when compared with an ACE inhibitor (enalapril).13,17 It also significantly improved symptoms. As such, patients with HFrEF who remain symptomatic should be transitioned from an ACE inhibitor or ARB to sacubitril/valsartan (class I recommendation, 2021 ESC guidelines2).
Patients hospitalised for decompensated HF are at high risk for further decompensation and death. Whilst the PARADIGM trial showed that sacubitril/valsartan reduced the risk of hospitalisation by 21% compared with enalapril, this study only recruited outpatients. Smaller, short-term trials have shown that hospitalised patients with acute HF can also be safely started on sacubitril/valsartan, including patients who are ACE inhibitor/ARB naïve.18,19
The PIONEER HF (Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NTproBNP in Patients Stabilized from an Acute Heart Failure Episode) trial18 compared initiation of sacubitril/valsartan versus enalapril in patients admitted to hospital with acutely decompensated HF, with the primary outcome at four and eight weeks being change in N-terminal pro-B-type natriuretic peptide (NTproBNP), a biomarker associated with higher risk of adverse outcomes. This trial showed sacubitril/valsartan was superior in reducing NTproBNP at all measured time points and was evident from the first week after initiation. Over half of the patients in this trial were ACE inhibitor/ARB naïve. Therefore, in carefully selected patients, an ARNI should be considered even in ACE inhibitor/ARB naïve patients (class IIb recommendation, 2021 ESC guidelines2). This should be an individualised decision and not all patients may be suitable for this, particularly if blood pressure is low.
Around the time sacubitril/valsartan received a license for the use in patients with HFrEF, trials looking at sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with type 2 diabetes reported favourable results with respect to HF outcomes. There followed two pivotal trials, DAPA-HF (Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction) and EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction), evaluating the benefit of dapagliflozin and empagliflozin in patients with HFrEF – with and without diabetes.14,16
The primary end point in both trials was a composite of CV mortality and hospitalisation for HF. Dapagliflozin reduced CV mortality and hospitalisation for HF by 26% compared with placebo, and empagliflozin by 25%. The benefit was observed whether the patients had diabetes or not. Both drugs significantly reduce the rate of decline in renal function. As a result, one of the major pharmacological changes in the new 2021 ESC HF guidelines is the incorporation of SGLT2 inhibitors as key disease-modifying drugs (class I recommendation, level A evidence). SGLT2 inhibitors appear well tolerated with little adverse impact on symptoms of hypotension. A single dose without need for up-titration makes this class of drug relatively straight-forward to use.
In summary, patients with HFrEF are at high risk of adverse outcome when the condition is not recognised in timely fashion or patients are not appropriately treated. There are now four pillars of pharmacological therapy to use as disease-modifying agents (figure 1). It is no longer considered necessary to start these sequentially and most would agree that it is imperative to start all four of these classes of drugs as quickly as possible to reduce the risk of HF hospitalisation, CV death and improve symptoms. The precise regimen will be influenced by the individual characteristics of the patient together with their wishes.
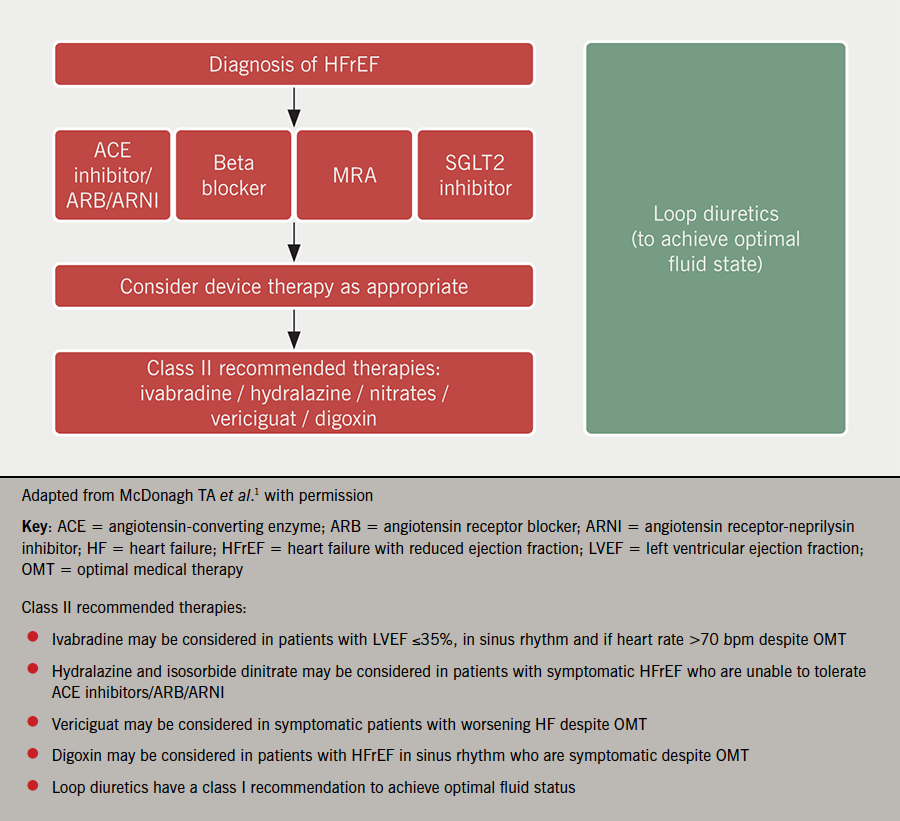
Pharmacological therapies for HFmrEF and HFpEF
Acknowledging the limited trial data for patients with HF with mildly reduced ejection fraction (HFmrEF) compared with those with HFrEF, the 2021 ESC guidelines have made treatment recommendations for these patients based on sub-group and post-hoc analysis of trials investigating patients with HFrEF and HF with preserved ejection fraction (HFpEF).
Patients with HFmrEF appear to behave more like HFrEF than HFpEF. Therefore, ACE inhibitors, ARBs, beta blockers, MRAs and ARNIs may be considered for the treatment of patients with HFmrEF to reduce the risk of death and hospitalisation for HF. All are given a class IIb recommendation, level C evidence2 to highlight the lack of specific evidence in this group of patients.
Patients with HFpEF generally tend to have different characteristics to those with a left ventricular ejection fraction (LVEF) ≤49%; they are more often female, with atrial fibrillation and have more non-cardiovascular co-morbidities. To date, no randomised controlled trials with ACE inhibitors, ARBs, ARNIs, MRAs and beta blockers have definitively shown a reduction in mortality or hospitalisation for HF in this population. Therefore, the 2021 ESC guideline only specifically recommends diuretics for use in this group of patients but highlights the importance of managing co-morbidities.
However, EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) published shortly after the new 2021 ESC guidelines compared empagliflozin with placebo in patients with LVEF ≥40% (i.e. patients with HFmrEF and HFpEF).20 This study showed there was a 21% reduction in CV mortality and hospitalisation for HF with empagliflozin compared with placebo (p<0.001), mainly driven by a reduction in hospitalisation for HF. The trial also showed a 36% reduction in the mean rate of decline in estimated glomerular filtration rate (eGFR) with empagliflozin (p<0.001). SGLT2 inhibitors are not currently licensed for HF use in patients with LVEF >40%, although it is anticipated that is likely to change in due course. Until then, they can be used according to their current license for treatment of diabetes and chronic kidney disease (CKD), both of which are extremely common co-morbidities.21,22
Heart failure and atrial fibrillation (AF)
Table 2. Recommendations for the use of oral anticoagulation in patients with heart failure and atrial fibrillation, according to their CHA2DS2-VASc score
| CHA2DS2-VASc score | Class of recommendation (level of evidence) |
|
|---|---|---|
| Women | ≥3 | I (A) |
| 2 | IIa (B) | |
| Men | ≥2 | I (A) |
| 1 | IIa (B) |
These two conditions commonly occur together. There is a strong recommendation that all patients with HF and AF are considered for oral anticoagulation if their CHA2DS2-VASc score is ≥1 in men and ≥2 in women (see table 2). A direct oral anticoagulation (DOAC) is the anticoagulation of choice due to lower risk of stroke, intracranial haemorrhage or death as compared with warfarin, unless a patient has a mechanical heart valve or moderate-severe mitral stenosis.
Regarding rate control, beta blockers and digoxin are both suitable (class IIa recommendation2) for chronic heart rate control in patients with HF, regardless of LVEF.23,24 Beta blockers have a class I indication as first-line treatment to control rate, assuming the patient is euvolaemic. If a patient presents with decompensated HF, caution should be given to the introduction of beta blockers until the patient is haemodynamically stable and their fluid state improved (in such patients, initial rate control can be achieved by digoxin and amiodarone, if required).
The latest 2021 ESC HF guidelines reiterate the need to consider appropriate use of rhythm control strategies in selected patients. The CASTLE AF (Catheter Ablation for Atrial Fibrillation with Heart Failure) trial recruited patients with an LVEF ≤35% with an implanted cardioverter defibrillator (ICD) who had paroxysmal or persistent AF. They were randomised to catheter ablation or standard care (which included rate or rhythm control).25 The trial concluded that catheter ablation was associated with a significantly reduced mortality and hospitalisation for HF but the overall number of patients achieving the primary end point was low and there was significant crossover between groups.
The 2021 ESC guidelines reflect this by suggesting patients with paroxysmal or persistent AF and worsening symptoms of heart failure associated with AF, despite medical therapy, should be considered for catheter ablation (class IIa recommendation) or direct current cardioversion (class IIb recommendation). Atrioventricular node ablation may be considered to control heart rate and relieve symptoms in those patients who do not tolerate or respond to pharmacological therapy. The majority of such patients will require cardiac resynchronisation therapy pacemaker/defibrillator and will be rendered pacemaker dependent.
Iron deficiency and anaemia
Iron deficiency (defined as ferritin <100 µg/L or 100–300 µg/L with transferrin saturation <20%) is common in patients with HF and independently predicts adverse outcome. Correction of iron deficiency with intravenous (IV) iron has been shown in the short term to improve quality of life and exercise capacity as compared with placebo. A meta-analysis recently showed potential impact on reduction in risk of HF hospitalisation.26
One of the trials in this meta-analysis, AFFIRM-AHF (Study to Compare Ferric Carboxymaltose with Placebo in Patients with Acute Heart Failure and Iron Deficiency), evaluated whether giving IV iron (ferric carboxymaltose) influenced CV mortality and hospitalisations for heart failure.27 The trial included patients with LVEF ≤50% during hospitalisation for acute HF and who were iron deficient. Patients were followed up for a year, but no IV iron was given beyond the first 24 weeks. Whilst the primary end point just failed to meet significance, patients who were given IV ferric carboxymaltose after stabilisation but prior to discharge had significantly fewer subsequent hospitalisations with HF. There did not, however, appear to be an effect on mortality.
Several longer-term studies, including the UK-based IRONMAN (Intravenous Iron Therapy in Patients with Heart Failure and Iron Deficiency) trial, will provide further data on long-term efficacy, safety and effect on CV death.28
On the basis of the trials above, the 2021 ESC HF guidelines recommend the following:
- Iron studies to be performed periodically in patients with HF (class I recommendation, and for those who are iron deficient).
- IV ferric carboxymaltose should be considered in symptomatic HF patients with LVEF ≤45% to improve symptoms (class IIa recommendation).
- IV ferric carboxymaltose should be considered in symptomatic, recently hospitalised HF patients with LVEF ≤50% to reduce risk of HF hospitalisation (class IIa recommendation).
Practical considerations when optimising drug therapy in HF patients
Starting SGLT2 inhibitors:
- DAPA HF and EMPEROR-Reduced both demonstrated that SGLT2 inhibitors do not cause a significant excess of symptomatic hypotension. This is an attractive drug class to use in patients with relatively low blood pressure.
- When initiating an SGLT2 inhibitor, a reduction in eGFR should be expected in the first few weeks after starting the drug (due to its effect on glomerular afferent arteriolar vasoconstriction). Thereafter, the eGFR typically plateaus and, in the long term, SGLT2 inhibitors slow the rate of decline in eGFR. As such they should not be stopped or held if the eGFR initially drops slightly.
- There is a small increased risk of genital infections, typically fungal infections, with SGLT2 inhibitor use due to increased urinary excretion of glucose. Patients should be counselled to maintain good levels of hygiene to try to avoid this.
- As SGLT2 inhibitors increase the concentration of glucose reaching the distal tubule, they also act as a diuretic. Occasionally patients may need a reduced dose of loop diuretics after starting these medications.
- In patients with diabetes, other hypoglycaemic medications should be reviewed in case they need adjustment when initiating SGLT2 inhibitors.
General considerations:
- An important feature of the 2021 ESC HF guidelines is the absence of hierarchy when initiating drugs to patients with a new diagnosis of HFrEF. This enables a more individualised, tailored approach to initiating management and should incorporate the patient’s view.
- Hypotension and the use of HF medications – if patients are on a stable dose and are asymptomatic, do not automatically stop/hold their disease-modifying drugs.
- Hyperkalaemia is a serious condition and can be caused by some HF drugs and exacerbated by common co-morbidities such as CKD. It is a common reason for stopping or not up-titrating some key drugs such as MRAs and other renin-angiotensin-aldosterone system (RAAS) blockers. Potassium-binding agents can be used in patients to lower potassium and enable these drugs to be prescribed. They have been shown in randomised controlled trials to be effective compared with placebo.29,30
Summary
The past few years have seen great progress with disease-modifying drugs for HF – yet many patients still do not receive these life prolonging treatments or have them inappropriately stopped. A multidisciplinary team approach is crucial to the success of managing this population of patients, particularly when it comes to monitoring, up-titration and tailoring doses for an individual patient. The 2021 ESC HF guidelines bring together the most up-to-date, evidence-based knowledge available for the treatment of HF in a user-friendly way for all clinicians. The key is to ensure that all patients living with HF have access to specialist care and the full armamentarium of treatments.
Key messages
- Dapagliflozin and empagliflozin have been given a class Ia recommendation for use in HFrEF and have an emerging role in those with HFmrEF and HFpEFs
- In HFrEF, the four pillars of heart failure therapy (ACE inhibitors/ARB/ARNI, MRA, beta blocker, SGLT2 inhibitors) should be started as soon as possible, tailored to the individual patient’s needs, and no longer need to be started sequentially
- More emphasis should be placed on the use of ARNI over the traditional ACE inhibitors/ARBs in appropriate patients
Acknowledgement
We thank Louis Graham-Hart for help with the figure.
Conflicts of interest
HH: none. PRK has received research and service improvement grants from AstraZeneca, Pharmacosmos, and Vifor Pharma; speaker/advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim, Napp, Novartis, Pharmacosmos, Servier, and Vifor Pharma.
Helen Hardy
Cardiology Specialist Registrar (ST7)
Paul R Kalra
Consultant Cardiologist
Portsmouth Hospitals University NHS Trust, Portsmouth, PO6 3LY.
Articles in this supplement
Introduction
New developments in the investigations and diagnosis of heart failure
Guidance on lifestyle, rehabilitation and devices in heart failure patients
References
1. Healthcare Quality Improvement Partnership. National Cardiac Audit Programme: National Heart Failure Audit (NHFA), 2021 Summary Report (2019/20 data). https://www.hqip.org.uk/resource/national-heart-failure-audit-nhfa-2021-summary-report/#.YgKM0cbLfUo (last accessed 8th February 2022)
2. McDonagh TA, Metra M, Adamo M et al. The ESC Scientific Document Group. (2021). 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
3. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study. N Engl J Med 1987;316:1429–35. https://doi.org/10.1056/NEJM198706043162301
4. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. https://doi.org/10.1056/NEJM199108013250501
5. Packer M, Poole-Wilson PA, Armstrong PW et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100:2312–18. https://doi.org/10.1161/01.CIR.100.23.2312
6. McMurray JJ, Ostergren J, Swedberg K et al., CHARM Investigators Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–71. https://doi.org/10.1016/S0140-6736(03)14283-3
7. Granger CB, McMurray JJ, Yusuf S et al., CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–6. https://doi.org/10.1016/S0140-6736(03)14284-5
8. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. https://doi.org/10.1016/S0140-6736(98)11181-9
9. Packer M, Coats AJ, Fowler MB et al., Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. https://doi.org/10.1056/NEJM200105313442201
10. Flather MD, Shibata MC, Coats AJ et al., SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–25. https://doi.org/10.1093/eurheartj/ehi115
11. Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–17. https://doi.org/10.1056/NEJM199909023411001
12. Zannad F, McMurray JJ, Krum H et al., EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. https://doi.org/10.1056/NEJMoa1009492
13. McMurray JJV, Packer M, Desai A S et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. https://doi.org/10.1056/NEJMoa1409077
14. McMurray JJV, Solomon SD, Inzucchi SE et al., DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
15. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. https://doi.org/10.1056/NEJMoa2024816
16. Packer M, Anker SD, Butler J, et al., EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190
17. Ponikowski P, Voors AA, Anker SD et al., ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128
18. Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–48. https://doi.org/10.1056/NEJMoa1812851
19. Wachter R, Senni M, Belohlavek J et al., on behalf of the TRANSITION Investigators. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019;21:998–1007. https://doi.org/10.1002/ejhf.1498
20. Anker SD, Butler J, Filippatos G et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038
21. https://www.medicines.org.uk/emc/product/5441/smpc#gref
22. https://www.medicines.org.uk/emc/medicine/27188#CONTRAINDICATIONS
23. Van Gelder IC, Rienstra M, Crijns HJGM, Olshansky B. Rate control in atrial fibrillation. Lancet 2016:388;818–28. https://doi.org/10.1016/S0140-6736(16)31258-2
24. Kotecha D, Bunting KV, Gill SK et al. Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) Team. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–508. https://doi.org/10.1001/jama.2020.23138
25. Marrouche NF, Brachmann J, Andresen D et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855
26. Graham FJ, Pellicori P, Ford I, Petrie MC, Kalra PR, Cleland JGF. Intravenous iron for heart failure with evidence of iron deficiency: a meta-analysis of randomised trials. Clin Res Cardiol 2021;110:1299–1307. https://doi.org/10.1007/s00392-021-01837-8
27. Ponikowski P, Kirwan BA, Anker SD et al.; AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396(10266):1895–1904. https://doi.org/10.1016/S0140-6736(20)32339-4
28. Clinicaltrials.gov. Intravenous Iron Treatment in Patients with Heart Failure and Iron Deficiency (IRONMAN). Clinical trials.gov Identifier: NCT02642562. https://clinicaltrials.gov/ct2/show/NCT02642562 (last accessed 8th Feburary 2022)
29. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011;32:820–8. https://doi.org/10.1093/eurheartj/ehq502
30. Pitt B, Bakris GL, Weir MR et al. Long-term effects of patiromer for hyperkalaemia treatment in patients with mild heart failure and diabetic nephropathy on angiotensin-converting enzymes/angiotensin receptor blockers: results from AMETHYST-DN. ESC Heart Fail 2018;5(4):592–602. https://doi.org/10.1002/ehf2.12292
