Untreated heart failure with reduced ejection fraction (HFrEF) has a one-year mortality rate of 40%. The DAPA-HF trial found that dapagliflozin reduces mortality and heart failure (HF) hospitalisation by 17% and 30%, respectively. We describe the initiation and real-world tolerability of dapagliflozin for the management of HFrEF at a large university teaching hospital in central London.
We reviewed 118 HFrEF patients initiated on dapagliflozin from January to August 2021 in both inpatient and outpatient settings using the Trust’s electronic records. A total of 69 (58.4%) patients were on optimised HF pharmacological therapy upon initiation of dapagliflozin. Dapagliflozin was discontinued in 12 (13.0%) patients. Twenty-three (42.6%) patients either discontinued or had a dose reduction in loop diuretics post-initiation of dapagliflozin.
In clinical practice, early initiation of dapagliflozin is safe, well-tolerated and resulted in earlier discontinuation or dose reduction in loop diuretics, providing opportunities to further optimise other HF medicines. This retrospective observational study supports the safety of the updated European Society of Cardiology (ESC) guidelines to initiate all four key HF medicines to minimise delays in HF treatment optimisation, which could translate to reduced National Health Service healthcare costs through fewer HF hospitalisations.
Introduction
The prevalence of heart failure (HF) in the UK is estimated to be 920,000, with 200,000 new diagnoses every year.1 HF is the most common cause of admission for people over 65 years old and accounts for 2% of the National Health Service (NHS) total budget, which is approximately £2 billion. Seventy per cent of these costs are attributed to HF hospitalisation, amounting to £3,796 per episode of HF hospital admission, based on an average length of stay of 13 days.2 Additionally, untreated heart failure with reduced ejection fraction (HFrEF) has a mortality rate of approximately 40%,3,4 therefore, evidence-based pharmacological treatments are the cornerstones for the management of HFrEF. The current standard of care, which is an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB) or angiotensin-receptor/neprilysin inhibitor (ARNI), beta blocker (BB) and mineralocorticoid-receptor antagonist (MRA), offer incremental benefits to HFrEF patients, including reducing one-year mortality to 6.4%, preventing hospitalisations, improving functional capacity and, subsequently, the quality of life, with an estimated additional five years gained.5,6 Oftentimes, the current treatments for HF are not adequately optimised or, in some cases, are underutilised.7 However, for optimal patient outcomes, patients need to be on optimised treatment dosages. Understandably, this is not always achievable in clinical practice as polypharmacy, comorbidities and patient tolerability pose a significant barrier to optimising pharmacological treatments.8 Therefore, there is an unmet demand for new treatments and a more simplified treatment algorithm to minimise delays in treatment optimisation.
In 2019, the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, found that dapagliflozin reduces mortality and HF-related hospitalisations by 17% and 30%, respectively.9 Additionally, clinical benefit of dapagliflozin was achieved as early as 28 days post-initiation.10 Subsequently, dapagliflozin became the first sodium-glucose co-transporter 2 (SGLT2) inhibitor to be licensed for the management of HFrEF independent of diabetes status. Evidence has shown that the combination of all four key HF therapies (ARNI, BB, MRA and SGLT2 inhibitor) provided the greatest benefits to HFrEF patients in reducing mortality and HF hospitalisation.11–13
The current National Institute for Health and Care Excellence (NICE) guidelines still follow the traditional medication sequencing strategy adopted from the landmark clinical trials, resulting in the use of dapagliflozin as an add-on therapy.14 The updated European Society of Cardiology (ESC) guidelines recognise the incremental and independent benefits of each foundational HF agent and support the simultaneous initiation of all HF pharmacological therapies to minimise delays in HF treatment optimisation.15 Consequently, there is wide variation in local policies regarding the initiation, monitoring and patient counselling for dapagliflozin in the management of HFrEF. This article evaluates the initiation and real-world tolerability of dapagliflozin for the management of HFrEF in a central London University teaching hospital population.
Methods
Study design and setting
This was a retrospective analysis involving 118 patients with HFrEF initiated on dapagliflozin in both the inpatient and outpatient settings at Guy’s and St. Thomas’ NHS Foundation Trust (GSTT) between January and August 2021. Data were collected, managed and analysed by the first author and critically reviewed by all the other authors to ensure accuracy and completeness of the data analysis.
All patients were required to have an echocardiogram or cardiac magnetic resonance imaging (MRI) assessment of left ventricular ejection fraction (LVEF) ≤40%, or confirmed HFrEF with undulating heart function/LVEF revised on consultant review if LVEF was >40%, and initiated on dapagliflozin for the management of HFrEF between January and August 2021 in either the inpatient or outpatient setting at GSTT. No patients with type 1 diabetes mellitus were included in this study.
Data collection
Table 1. Patient demographics for this cohort
| Characteristic | N=118 |
|---|---|
| Mean age ± SD, years | 66 ± 13 |
| Mean LVEF ± SD, % | 28 ± 9 |
| Gender, n (%) | |
| Male | 85 (72.0) |
| Female | 33 (28.0) |
| Ethnicity, n (%) | |
| White | 72 (61.0) |
| Black | 18 (15.3) |
| Other | 10 (8.5) |
| Not stated | 18 (15.3) |
| Inpatient/outpatient initiation of dapagliflozin, n (%) | |
| Inpatient | 58 (49.2) |
| Outpatient | 56 (47.5) |
| Outpatient telephone clinic | 4 (3.4) |
| Comorbidity, n (%) | |
| Type 2 diabetes mellitus | 36 (30.5) |
| Atrial fibrillation | 43 (36.4) |
| Presence of cardiac device, n (%) | 60 (50.8) |
| ICD | 22 (18.6) |
| CRT-D | 32 (27.1) |
| CRT-P | 4 (3.4) |
| Dual-chamber pacemaker | 2 (1.7) |
| Key: CRT-D = cardiac resynchronisation therapy with a defibrillator; CRT-P = cardiac resynchronisation therapy pacemaker; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; SD = standard deviation | |
A data collection tool was created using Microsoft® Excel 2021 with password protection to collect patient data. Demographic data, clinical parameters, laboratory results, and medications upon initiation of dapagliflozin, and an average of three months post-initiation of dapagliflozin, were extracted from the Trust’s electronic records. Each patient was also given a unique patient identifier to maintain confidentiality. The decision on whether patients were on optimised pharmacological therapy was made by the HF pharmacist (GC) based on their professional judgement and following the South East London Guidance on the Pharmacological Management of Heart Failure in Adults.
Statistical analysis
Descriptive statistics and non-parametric tests using IBM® SPSS® Statistics version 27 were employed for the analysis of the results of this study. Categorical data were presented as number of patients and percentages; normally distributed, continuous data as means ± standard deviations (SD); non-normally distributed, continuous clinical parameters as medians and interquartile ranges (IQR). The non-parametric Wilcoxon signed-rank test and Hodges-Lehmann estimator were employed due to the small sample size of the study resulting in non-normally distributed results, statistical significance was defined as a two-tailed value of p<0.05.
Results
Baseline patient characteristics
A total of 118 patients were initiated on dapagliflozin at GSTT between January and August 2021. The mean age was 66 ± 13 years, 72.0% (n=85) were male, 30.5% (n=36) had type 2 diabetes mellitus (T2DM) and 36.4% (n=43) had atrial fibrillation (table 1).
Clinical parameters
The median time interval for follow-up, renal function and glycated haemoglobin (HbA1c) tests was three months (IQR 8–16 weeks), three months (IQR 8–12 weeks) and 2.5 months (IQR 8–12 weeks), respectively. A total of 92 (78.0%) patients had a follow-up and 26 (22.0%) patients were lost to follow-up in that these patients had no further appointments booked with the Trust. A total of 58.4% (n=69) of patients were on optimised HF pharmacological therapy upon initiation of dapagliflozin. There was a decrease in systolic blood pressure by 5 mmHg (p=0.025), HbA1c by 2.0 mmol/mol (p=0.026) and weight by 1.4 kg (p=0.007) from baseline. However, there were no significant differences between the baseline and follow-up in serum potassium level, heart rate and renal function (table 2).
Table 2. Clinical parameters for patients in this cohort measured at baseline and follow-up
| Clinical parameter | No. of patients checked at baseline (%) N=118 | Baseline median (IQR) | No. of patients checked at follow-up (%) N=92 | Follow-up median (IQR) | Estimated median change from baseline (95%CI) | Estimated median change from baseline (95%CI) |
|---|---|---|---|---|---|---|
| HbA1c, mmol/mol | 80 (67.8) | 42 (40–53) | 42 (45.6) | 42 (38–54) | –2.0 (–5.0 to 0.0) | 0.026 |
| Systolic blood pressure, mmHg | 114 (96.6) | 118 (106–135) | 86 (93.5) | 111 (100–131) | –5.0 (–9.5 to –0.5) | 0.025 |
| Heart rate, bpm | 114 (96.6) | 73 (63–80) | 86 (93.5) | 69 (62–77) | –1.5 (–4.5 to 1.0) | 0.233 |
| Serum potassium, mmol/L* | 114 (96.6) | 4.5 (4.2–5.0) | 89 (96.7) | 4.6 (4.3–4.9) | +0.05 (–0.1 to 0.2) | 0.366 |
| Weight, kg | 114 (96.6) | 80.5 (63.3–94.9) | 85 (92.4) | 78.6 (65.0–88.9) | –1.4 (–2.5 to –0.4) | 0.007 |
| Renal function | 114 (96.6) | 89 (96.7) | ||||
| eGFR, ml/min/1.73 m2 | 58 (45–75) | 59 (46–73) | –1.0 (–3.0 to 1.5) | 0.503 | ||
| Serum creatinine, µmol/L | 106 (85–128) | 102 (86–126) | +1.5 (–2.5 to 5.0) | 0.493 | ||
| *All data except serum potassium levels were non-normally distributed and, therefore, do not meet the assumptions of a parametric test. The median of the serum potassium level was used to simplify data reporting. Key: CI = confidence interval; eGFR = estimated glomerular filtration rate; HbA1c = glycated haemoglobin; IQR = interquartile range |
||||||
Changes to HF medications
More patients were on ACEi/ARB as compared with ARNI upon the initiation of dapagliflozin (figure 1). However, 17 (18.5%) patients on ACEi/ARB at baseline were switched to ARNI during their three-month follow-up. Overall, most patients were able to remain on the same dose for all HF medications post-initiation of dapagliflozin (table 3).
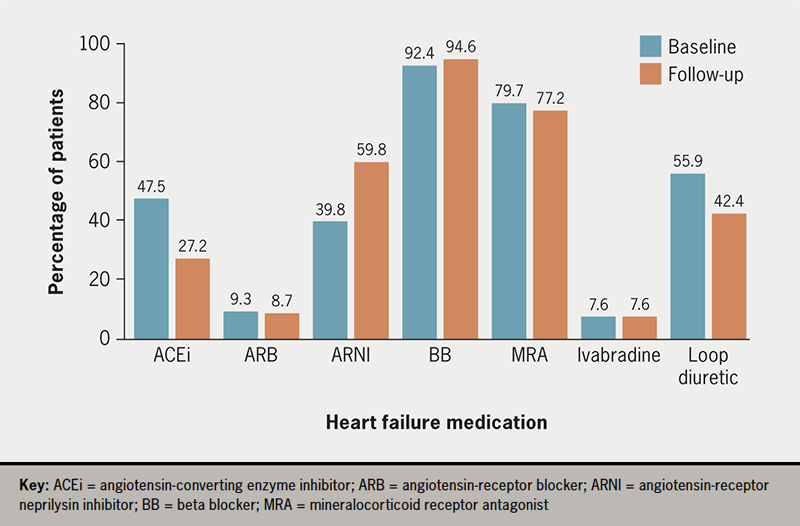
Table 3. Changes to patients’ heart failure medications post-initiation of dapagliflozin
| Heart failure medication | Dose decreased | Dose increased | Switched to ARNI | Newly added | Discontinued |
|---|---|---|---|---|---|
| ACEi/ARB | 5 (5.4) | 4 (4.3) | 17 (18.5) | 1 (1.1) | 3 (3.3) |
| ARNI | 5 (5.4) | 8 (8.7) | – | 0 (0.0) | 1 (1.1) |
| BB | 8 (8.7) | 9 (9.8) | – | 1 (1.1) | 0 (0.0) |
| MRA | 3 (3.3) | 7 (7.6) | – | 0 (0.0) | 6 (6.5) |
| Ivabradine | 1 (1.1) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) |
| All data expressed as number of patients (%), where N=92 Key: ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin-receptor/neprilysin inhibitor; BB = beta blocker; MRA = mineralocorticoid receptor antagonist |
|||||
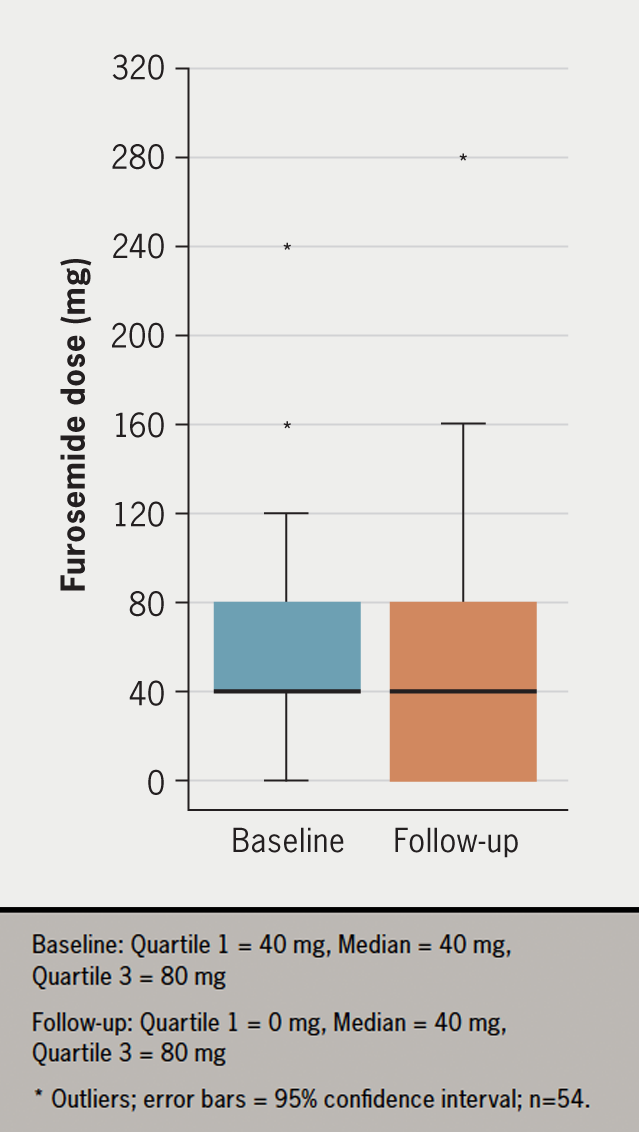
During the three-month follow-up, 42.6% (n=23) of patients either discontinued or had a dose reduction in loop diuretics, while 48.1% (n=26) of patients remained on the same dose (figure 2). For patients taking bumetanide, the furosemide-equivalent dose was calculated and used to simplify the reporting of the results. The estimated median reduction in furosemide dose post-initiation of dapagliflozin was 20 mg (95% confidence interval [CI] –20 to 0, p=0.001).
Safety and tolerability of dapagliflozin
Dapagliflozin was discontinued for reasons other than death in 12 (13.0%) patients. Of the 12 patients, dapagliflozin was temporarily discontinued in two (2.2%) patients. The main reasons dapagliflozin was discontinued were genital thrush infections (n=3, 25.0%), not tolerated for other reasons (n=2, 16.7%) and unacceptable renal insufficiency (n=2, 16.7%). Other reasons (n=5, 41.7%) included increased dyspnoea, hypotension, dehydration, recurrent urinary tract infection and polyuria.
Discussion
The present study highlights the potential benefits of the early initiation of dapagliflozin, particularly the changes to other HF pharmacological treatments, and the tolerability of dapagliflozin. The early initiation of dapagliflozin in this study led to the discontinuation or dose reduction in loop diuretics in 42.6% (n=23) of patients at three months. This is higher than the results from the DAPA-HF trial, which only initiated dapagliflozin in patients when treatment had already been optimised, where only 10.4% of patients had a dose reduction in diuretics at six months.16 Interestingly, both the DAPA-HF and Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction (EMPEROR-Reduced) trial also found that fewer patients in the treatment group required intensification of diuretic treatment during their follow-up.10,16 These are significant benefits as loop diuretics are used only to relieve congestion symptoms and improve exercise capacity without reducing mortality.17 Additionally, patients who remain on diuretics long term, particularly on high doses, are associated with higher mortality, which may indicate the presence of more severe disease.18 Therefore, reduction in diuretic dose and relief from congestion without the need for long-term diuretics is prognostically advantageous for HF patients.
The discontinuation of loop diuretics may also provide opportunities for clinicians to further optimise other disease-modifying HF medicines. The present study found that 18.5% (n=17) who were on ACEi/ARB at baseline switched to ARNI three months post-initiation of dapagliflozin. The superiority of ARNI to ACEi in reducing mortality and HF hospitalisation has been documented in several studies.11,12,19,20 While this study found reductions in blood pressure and weight, these changes may result from the concomitant up-titration of other HF therapies and not just the effect of dapagliflozin alone.
The results of the present study indicate that dapagliflozin is safe and well-tolerated by HFrEF patients with early use in combination with other prognostic therapies. The discontinuation rate of dapagliflozin in this study was similar to the other SGLT2 inhibitor studies.9,20,21 There were several reasons dapagliflozin was discontinued permanently in these patients, with the main reason being recurrent genital thrush infections. Similar reasons have also been reported in previous trials, where a higher incidence of genital fungal infections was found in the treatment group, resulting in the discontinuation of dapagliflozin.22-24 Proper counselling on perineal hygiene, signs and symptoms of genital fungal infections, and sick day rules are required to avoid discontinuation of dapagliflozin.
Ultimately, the greatest benefits will be achieved at a population level if SGLT2 inhibitors can be safely initiated and managed collaboratively in primary and secondary care, given their benefits in treating and preventing multiple conditions, including HF, T2DM and chronic kidney disease with proteinuria.9,25-27 Many patients affected by these conditions may never require referral to secondary care. Hence, there is a need for simplification of a practical guideline to cover initiation and management for a variety of indications, with universal application in primary and secondary care, to avoid unnecessary variation in practice and confusion. HF patients, particularly those with heart failure with preserved ejection fraction (HFpEF), will also benefit from the approval of other SGLT2 inhibitors, such as empagliflozin, which was recently approved for the treatment of HFrEF and HFpEF, as this will extend the treatment options for patients across the HF spectrum.25,28
Limitations
This study has a number of limitations worthy of mention. First, the study was a single-centre, retrospective, observational study with a relatively small sample size, which could affect the generalisability of the results. Second, there were a few differences between the patient demographic at GSTT and the rest of the UK, as London has a significantly higher ethnic diversity. The mean age in this study is younger than the average age of HF diagnosis in the NHS (66 vs. 77 years).14 Seventy-two per cent of the patients (n=85) recruited in this study were male, 61.0% (n=72) of the cohort were white and 15.3% (n=18) were black.14 Therefore, the population in the study may not reflect other local HFrEF populations across the UK.
In this study, some patients did not have a follow-up as they were discharged back to their local communities outside of South East London, where we have restricted access to patient data. Besides that, the follow-up timeframes were inconsistent due to the nature of the appointment booking system within the Trust.
Finally, and most importantly, the effects described in the present study are not to be attributed to dapagliflozin alone, as concomitant up-titration of other HF medicines and treatment responses related to cardiac devices also occurred simultaneously. Therefore, the outcomes measured may represent changes resulting from the composite effects of therapy with these potential confounding factors, and not purely the effect of the initiation of dapagliflozin alone.
Conclusion
In conclusion, this study has demonstrated the benefits and safety of the early initiation of dapagliflozin in patients with HFrEF in line with the updated ESC HF guideline. In clinical practice, early initiation of dapagliflozin resulted in earlier discontinuation or dose reduction in loop diuretics, providing opportunities to further optimise other HF medicines. Dapagliflozin is safe and well-tolerated in a real-world population, outside the setting of a clinical trial. Counselling on perineal hygiene may avoid discontinuation of dapagliflozin due to minimising the risk of genital thrush infection. This study supports the safety of the updated ESC guideline position to initiate all four key HF medicines concurrently, without stepwise maximal up-titration and sequential addition. This new treatment algorithm will minimise delays in HF treatment optimisation, which should translate to reduced NHS healthcare costs through fewer HF hospitalisations and, as such, increase hospital inpatient capacity with greater bed availability.
Key messages
- The early initiation of dapagliflozin resulted in the early discontinuation or dose reduction in loop diuretics, providing opportunities for clinicians to further optimise other heart failure (HF) medications
- Dapagliflozin is safe and well-tolerated in the real-world setting
- Dapagliflozin should be considered as a key HF pharmacological therapy rather than an add-on therapy, hence, it should be initiated earlier in the treatment pathway in order to minimise delays in treatment optimisation.
Conflicts of interest
None declared.
Funding
None.
Study approval
As this was a service evaluation, no ethical approval was needed. The evaluation was registered on the Trust’s audit database. Patient consent was not required.
Acknowledgements
Thank you to the heart failure team at Guy’s and St. Thomas’ Hospital for the care of these patients and support of this work. We would particularly like to acknowledge Professor Gerald Carr-White, Dr Jessica Webb, Dr Tevfik F Ismail, Dr Stamatis Kapetanakis, Dr Laura-Ann McGill and Dr Syed Gardezi.
References
1. British Heart Foundation. Heart & Circulatory Disease Statistics 2021. London: BHF, 2021. Available from: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics [accessed 7 November 2021].
2. British Heart Foundation. An integrated approach to managing heart failure in the community. London: BHF, 2015. Available from: https://www.heartfailurehubscotland.co.uk/wp-content/uploads/2016/04/HF-an-integrated-approach-to-managing-heart-failure-in-the-community-sirhf1.pdf
3. Cowie MR, Wood DA, Coats AJS et al. Survival of patients with a new diagnosis of heart failure: a population-based study. Heart 2000;83:505–10. https://doi.org/10.1136/heart.83.5.505
4. Ho KKL, Anderson KM, Kannel WB et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–15. https://doi.org/10.1161/01.CIR.88.1.107
5. Shah A, Gandhi D, Srivastava S et al. Heart failure: a class review of pharmacotherapy. P T 2017;42:464. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5481297/
6. Crespo-Leiro MG, Anker SD, Maggioni AP et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–25. https://doi.org/10.1002/ejhf.566
7. National Institute for Cardiovascular Outcomes Research. Heart Failure. Available at: https://www.nicor.org.uk/category/heart-failure/ [accessed 17 December 2021].
8. Marti CN, Fonarow GC, Anker SD et al. Medication dosing for heart failure with reduced ejection fraction – opportunities and challenges. Eur J Heart Fail 2019;21:286–96. https://doi.org/10.1002/ejhf.1351
9. McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med 2019;381:1995–2008. https://doi.org/101056/NEJMoa1911303
10. Tomasoni D, Fonarow GC, Adamo M et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2022;24:431–41. https://doi.org/10.1002/ejhf.2397
11. Tromp J, Ouwerkerk W, van Veldhuisen DJ et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2022;10:73–84. https://doi.org/10.1016/j.jchf.2021.09.004
12. Vaduganathan M, Claggett BL, Jhund PS et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121–8. https://doi.org/10.1016/S0140-6736(20)30748-0
13. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J 2021;42:681–3. https://doi.org/10.1093/eurheartj/ehaa1012
14. National Institute for Health and Care Excellence. Dapagliflozin for treating chronic heart failure with reduced ejection fraction. TA679. London: NICE, 2021. Available from: https://www.nice.org.uk/guidance/ta679
15. McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC 2021. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
16. Jackson AM, Dewan P, Anand IS et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 2020;142:1040–54. https://doi.org/10.1161/CIRCULATIONAHA.120.047077
17. Casu G, Merella P. Diuretic therapy in heart failure – current approaches. Eur Cardiol Rev 2015;10:42. https://doi.org/10.15420/ecr.2015.10.01.42
18. Hasselblad V, Stough WG, Shah MR et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007;9:1064. https://doi.org/10.1016/j.ejheart.2007.07.011
19. Okumura N, Jhund PS, Gong J et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail 2016;9:e003212. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003212
20. Packer M, Anker SD, Butler J et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190
21. Bhatt DL, Szarek M, Steg PG et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. https://doi.org/10.1056/NEJMoa2030183
22. Wiviott SD, Raz I, Bonaca MP et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. https://doi.org/10.1056/NEJMoa1812389
23. Shah SR, Ali A, Ikram S. Sotagliflozin and decompensated heart failure: results of the SOLOIST-WHF trial. Expert Rev Clin Pharmacol 2021;14:523–5. https://doi.org/10.1080/17512433.2021.1908123
24. Anker SD, Butler J, Filippatos G et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation 2021;143:337–49. https://doi.org/10.1055/s-0041-1727472
25. Anker SD, Butler J, Filippatos G et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038
26. Zinman B, Wanner C, Lachin JM et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. https://doi.org/10.1056/NEJMoa1504720
27. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. https://doi.org/10.1056/NEJMoa2024816
28. Heidenreich PA, Bozkurt B, Anguilar D et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–e421. https://doi.org/10.1016/j.jacc.2021.12.012
