Surgical aortic valve replacement (SAVR) prolongs life and improves its quality in patients with severe aortic stenosis (AS). Unplanned SAVR is a failure of AS screening and follow-up programmes. We identified all elective, first, isolated SAVRs performed between 1 January and 31 December 2019 in a Welsh tertiary cardiac centre, and documented the clinical and echocardiographic variables, and reasons for unplanned SAVR.
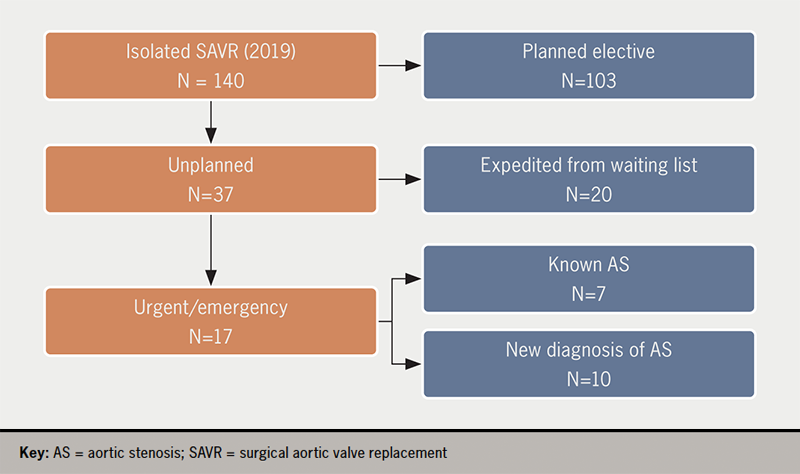
Of 140 isolated SAVR, 37 (26%) were unplanned (16 female, mean age 72.3 ± 8.4 years). Twenty had been on the SAVR waiting list and had expedited operations because of concerns about the severity of the AS (12 patients), or because of acute (four patients) or chronic (four patients) left ventricular failure (LVF). Of the 17 not on the waiting list, AS was known in seven: three had acute pulmonary oedema while under follow-up with ‘moderate AS’, one had been referred but developed pulmonary oedema while waiting for a surgical outpatient appointment, one refused SAVR but was subsequently admitted with acute pulmonary oedema and accepted SAVR, one was admitted directly from home because concerns about worsening AS, and one had infective endocarditis with severe aortic regurgitation. Of 10 patients with a new diagnosis of AS, five presented with LVF, four with angina and in three there was a history of syncope (p=0.003 vs. known AS; multiple symptoms). Survival, age, Canadian Cardiovascular Society (CCS) and New York Heart Association (NYHA) class, number of risk factors, peak and mean aortic valve (AV) gradients, AV area, and stroke volume index were not different between patients who had planned versus unplanned SAVR, or with known or new AS. Patients with a new diagnosis of AS had longer pre-operative wait (22.3 ± 9.3 vs. 6.0 ± 10.3 days, p<0.001).
In conclusion, a quarter of SAVRs are unplanned and half are in patients without a prior diagnosis of AS. Unplanned SAVR is associated with prolonged length of hospital stay and with a history of syncope, but other conventional clinical and echocardiographic parameters do not differ between patients undergoing planned versus unplanned SAVR.
Introduction
Surgical aortic valve replacement (SAVR) is the best established treatment for severe, symptomatic aortic valve stenosis (AS), where it restores life-expectancy to levels seen in the general population.1 Ideally, patients with AS should be followed-up in a valve clinic, so that the optimal timing for performing SAVR can be determined, based on a combination of periodically assessed symptoms, signs, imaging and laboratory tests.2,3 Operating too late carries an increased risk of death and of peri-operative complications, related to (potentially irreversible) deterioration of left ventricular (LV) function from afterload mismatch.4
From this perspective, any instance of SAVR performed as an unplanned procedure is a missed opportunity to provide timely care with the minimal possible risk.
We set out to analyse urgent, unplanned SAVR procedures in our centre, in an effort to understand factors that explain why some patients ‘slip through the net’ and are operated on in an unplanned manner, to help us determine what can be done to avoid this in the future.
Method
Setting
Morriston cardiac centre provides tertiary cardiology and cardiac surgical care to a population of about one million in West Wales, UK; there are five cardiac surgeons operating in two cardiac theatres, performing approximately 750 open-heart procedures annually.
Inclusion and exclusion criteria
We selected all unplanned (urgent or emergency) isolated, first SAVR procedures performed for AS in 2019, the last full year before the COVID-19 pandemic. We excluded SAVRs that had associated procedures, such as coronary artery bypass graft (CABG), root replacement, or concomitant intervention of any kind for another heart valve pathology.
Data analysis
The urgency of the operation was ascertained from our electronic surgical database (Dendrite Medical, Reading, UK). We also used the Welsh Clinical Portal to document patient- and procedure-related variables, as well as the pathway each patient followed between diagnosis and urgent/emergency SAVR, in order to identify factors that may have led to unplanned SAVR. Specifically, we documented presence and severity of AS symptoms on admission, whether patients were on a waiting list for SAVR at the time of their unplanned operation, whether the diagnosis of AS pre-dated the admission or not, which of the major cardiovascular risk factors were present, echocardiographic descriptors of the severity of the AS (peak aortic velocity, mean aortic gradient, aortic valve area by continuity, stroke volume index) and LV ejection fraction (LVEF) on the transthoracic echo closest to the date of SAVR, stage of AS-related myocardial damage,5 time lag between admission and operation, and the length of total and of postoperative hospital stay, as well as survival status (with a censoring date of 21 December 2021). We compared clinical and echo parameters between patients on, and those not on, the SAVR waiting list, and between patients with known AS versus those with a new diagnosis of AS during the index admission, using the Chi-square statistic for nominal variables and Student’s t statistic for ordinal, continuous variables; p<0.05 was deemed significant. Where the assumption of equal variance was violated we used the Welch and Mann-Whitney tests. We used JASP 0.16.0.0, an open-source statistical package, (https://jasp-stats.org/) for the statistical analysis.
Results
Patients
In 2019 there were 103 elective, first-time, isolated SAVRs and 37 (16 female, mean age 72.3 ± 8.4 years) non-elective, isolated, first SAVR (26% of total 140 SAVRs). Of these, 20 patients were already on the waiting list for SAVR and 17 were not (figure 1).
Clinical characteristics
Clinical characteristics of the patients are summarised in table 1, which is classified by patients on the waiting list versus patients not on the waiting list, and patients with a pre-existing diagnosis of AS versus patients without such a diagnosis. Frequency and severity of angina and of heart failure, prevalence of risk factors (table 2), aortic valve area, mean gradient and peak velocity, as well as stroke volume index and LVEF, were not different between those who were on the waiting list for SAVR, compared with those who were not, although LVEF was numerically lower and mean gradient numerically higher in patients who were not on the waiting list. The same findings apply to patient groups defined by whether a diagnosis of AS was known before the index admission. Almost one in three patients without a pre-existing diagnosis of AS presented with syncope, a much higher proportion than in those with known AS, but the significance of this finding is uncertain given the small size of our sample.
Table 1. Clinical and echocardiographic characteristics of patients stratified according to whether they were on the surgical waiting list for surgical aortic valve replacement (SAVR) or not, and whether they had been diagnosed with aortic stenosis (AS) or not prior to the index admission for SAVR. Variables in bold achieved statistical significance for the difference between the groups
| Variable, mean ± SD | Not on W/L for SAVR | On W/L for SAVR | p | AS not known | Known AS | p |
|---|---|---|---|---|---|---|
| Age at admission, years | 72.4 ± 8.7 | 72.2 ± 8.3 | 0.98 | 72.8 ± 9.1 | 72.1 ± 8.3 | 0.84 |
| CCS angina class | 1.6 ± 0.9 | 1.6 ± 0.8 | 0.87 | 1.4 ± 0.7 | 2.7 ± 0.8 | 0.04 |
| NYHA heart failure class | 2.8 ± 0.9 | 2.6 ± 0.7 | 0.40 | 2.7 ± 0.9 | 2.7 ± 0.8 | 0.80 |
| Number of cardiac risk factors | 1.7 ± 0.8 | 1.8 ± 1.1 | 0.80 | 1.9 ± 0.7 | 1.7 ± 1.1 | 0.75 |
| Peak velocity across AV, m/s | 4.5 ± 0.8 | 4.1 ± 0.9 | 0.18 | 4.5 ± 0.8 | 4.1 ± 0.9 | 0.31 |
| Mean aortic valve gradient, mmHg | 50.1 ± 20.0 | 40.8 ± 19.3 | 0.15 | 50.5 ± 19.7 | 43.1 ± 20.0 | 0.31 |
| Aortic valve area, cm2 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.55* | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.43 |
| LV ejection fraction, % | 49.4 ± 13.3 | 54.2 ± 10.3 | 0.22 | 52.2 ± 11.3 | 51.9 ± 12.3 | 0.95 |
| Stroke volume index, ml/m2 |
34.5 ± 7.6 | 33.2 ± 8.2 | 0.61 | 33.4 ± 7.7 | 33.9 ± 8.0 | 0.87 |
| Pre-operative hospital stay, days | 20.0 ± 11.8 | 2.2 ± 4.3 | 0.001* | 22.3 ± 9.3 | 6.0 ± 10.3 | 0.001 |
| Postoperative hospital stay, days | 16.4 ± 16.2 | 9.4 ± 6.7 | 0.008 | 12.5 ± 10.0 | 12.7 ± 3.3 | 0.9 |
| Total hospital stay, days | 36.5 ± 21.9 | 11.7 ± 7.7 | 0.01* | 34.8 ± 13.9 | 18.77 ± 20.4 | 0.029 |
| *Levene’s test was significant (p<0.05), suggesting a violation of the equal variance assumption, but p values remained the same with Welch’s and Mann-Whitney’s tests. Key: AS = aortic stenosis; AV = aortic valve; CCS = Canadian Cardiac Society; LV = left ventricle; NYHA = New York Heart Association; SAVR = surgical aortic valve replacement; SD = standard deviation; W/L = waiting list |
||||||
Table 2. Distribution by gender, main symptom at presentation and cardiovascular risk factors according to whether patients were on the waiting list for SAVR or not and whether patients were diagnosed with AS before presentation or not
| Patient characteristics and symptoms | Not on W/L for SAVR | On W/L for SAVR | p | AS not known | Known AS | p | |
|---|---|---|---|---|---|---|---|
| Gender | F | 9 | 7 | 0.27 | 5 | 11 | 0.60 |
| M | 8 | 13 | 5 | 16 | |||
| Angina | No | 12 | 16 | 0.50 | 6 | 22 | 0.17 |
| Yes | 5 | 4 | 4 | 5 | |||
| Heart failure | No | 8 | 6 | 0.28 | 6 | 8 | 0.09 |
| Yes | 9 | 14 | 4 | 19 | |||
| Syncope | No | 14 | 20 | 0.05 | 7 | 27 | 0.03 |
| Yes | 3 | 0 | 3 | 0 | |||
| T2DM | No | 12 | 12 | 0.50 | 8 | 16 | 0.24 |
| Yes | 5 | 8 | 2 | 11 | |||
| High BP | No | 7 | 7 | 0.62 | 2 | 12 | 0.62 |
| Yes | 10 | 13 | 8 | 14 | |||
| Current smoking | No | 15 | 20 | 0.11 | 9 | 26 | 0.45 |
| Yes | 2 | 0 | 1 | 1 | |||
| Family history of CAD | No | 16 | 17 | 0.3 | 10 | 23 | 0.3 |
| Yes | 1 | 3 | 0 | 4 | |||
| Chronic renal disease | No | 14 | 18 | 0.4 | 9 | 23 | 0.70 |
| Yes | 3 | 2 | 1 | 4 | |||
| Key: AS = aortic stenosis; BP = blood pressure; CAD = coronary artery disease; SAVR = surgical aortic valve replacement; T2DM = type 2 diabetes mellitus; W/L = waiting list | |||||||
Length of hospital stay
Length of hospital stay was significantly longer in patients who were not on the waiting list: total length of stay (mean of 36.5 ± 21.9 days vs. 11.7 ± 7.7 days, p<0.01); pre- and post-operative length of stay was also longer in patients not on the waiting list (table 1). Findings were similar for a comparison between patients with, versus those without, a pre-existing diagnosis of AS (table 1).
Patient pathways to SAVR
Patients with a previous diagnosis of AS
There were 27 patients with known AS. Of these, 20 were on the waiting list and seven were not. Reasons leading to expedited SAVR in the 20 patients on the waiting list included concern about how severe the AS was (12 patients – operation brought forward by the surgeon, after the initial surgical clinic appointment), and the development of a clinical emergency in eight: four cases of acute pulmonary oedema and four cases of congestive heart failure. Of seven patients not on the waiting list, three had acute pulmonary oedema while under routine cardiology follow-up for ‘moderate AS’, one had been referred but developed pulmonary oedema while waiting for a surgical outpatient appointment, one refused SAVR but was subsequently admitted with acute pulmonary oedema, one was admitted directly from home because of concerns from the GP about the severity of AS, and one had infective endocarditis on a background of mild AS, and was admitted with severe acute aortic regurgitation.
Patients without a previous diagnosis of AS
In 10 patients the diagnosis of AS was first established during the admission that led to SAVR: five presented with LV failure, four with angina and in three there was a history of syncope (p=0.003 vs. known AS; total >10 as some patients had multiple symptoms).
Table 3. Survival status according to whether patients were on the waiting list or not, and to whether they had been diagnosed with AS before the index admission
| Not on W/L for SAVR | On W/L for SAVR | p | AS not known | Known AS | p | |
|---|---|---|---|---|---|---|
| Alive | 15 | 16 | 0.49 | 9 | 22 | 0.53 |
| Dead | 2 | 4 | 1 | 5 | ||
| Key: AS = aortic stenosis; SAVR = surgical aortic valve replacement; W/L = waiting list | ||||||
Table 4. Time between initial diagnosis of AS and SAVR in years and the number of patients with and without a prior diagnosis of AS, according to whether patients were on the waiting list or not
| Not on W/L for SAVR | On W/L for SAVR | |
|---|---|---|
| Time between initial diagnosis of AS and SAVR in years (p=0.49) | ||
| N | 17 | 20 |
| Mean ± SD | 1.8 ± 2.3 | 2.4 ± 2.3 |
| Minimum | 0.0 | 0.2 |
| Maximum | 5.8 | 8.9 |
| Prior diagnosis of AS (p<0.001) | ||
| No | 10 | 0 |
| Yes | 7 | 20 |
| Total | 17 | 20 |
| Key: AS = aortic stenosis; SAVR = surgical aortic valve replacement; SD = standard deviation; W/L = waiting list | ||
Both patients not on the waiting list and those with a new diagnosis of AS had numerically worse indices of AS, but the differences were not significant. There was no difference in mortality, or in the stage of AS-related myocardial damage at presentation between patients on, or not on, the waiting list, or those with a prior versus a new diagnosis of AS (tables 3, 4 and 5). The extent of AS-related myocardial damage was not different between survivors and deceased (table 5).
Discussion
In a tertiary UK cardiac centre serving a population of one million, 12% of SAVR (17 of 140) were urgent, unplanned, and had increased pre-operative length of hospital stay. Almost half of these (7/17, 44%) unplanned SAVRs were in patients without a previous diagnosis of AS.
Another 20 operations (14% of SAVR) were expedited from the waiting list. These were patients who had been accepted for SAVR, but whose operations were brought forward by surgeons concerned about the severity of the disease, or who decompensated with heart failure while waiting for their procedure. For service improvement, studying patients who had unplanned operations is likely to be most informative.
Patients with operations expedited while on the waiting list for SAVR
Patients on the waiting list for SAVR have had pre-operative investigations completed and, in principle, could receive their SAVR at any time after they have been placed on the waiting list. If the NHS had the capacity to perform timely operations, this category of patients would not exist, or their number would be negligible. Limited capacity means that – unavoidably – some patients will decompensate while waiting for elective SAVR.
Table 5. Stage of myocardial damage by whether patients were on the waiting list for SAVR, had known AS, and mortality
| Stage of myocardial damage | Not on W/L for SAVR | On W/L for SAVR | AS not known | Known AS | Alive | Dead |
|---|---|---|---|---|---|---|
| 0 | 3 | 2 | 2 | 3 | 5 | 0 |
| 1 | 4 | 2 | 7 | 9 | 11 | 0 |
| 2 | 6 | 9 | 4 | 11 | 11 | 4 |
| 3 | 4 | 1 | 2 | 3 | 3 | 2 |
| 4 | 0 | 1 | 0 | 1 | 1 | 0 |
| Total (N=37) | 17 | 20 | 10 | 27 | 31 | 6 |
| p value | 0.37 | 0.79 | 0.16 | |||
| Key: AS = aortic stenosis; SAVR = surgical aortic valve replacement; W/L = waiting list | ||||||
In our sample of 37 patients, 20 were identified by their physicians as needing an expedited operation, and thus they ‘jumped the queue’ to an early SAVR, which is a compromise solution for the delivery of effective care within a constrained resources setting. Broadly speaking, these patients can be considered a ‘surrogate’ for the larger group of patients having genuinely elective, planned procedures, which do not need to be brought forward. However, of these 20, only 12 were clinically stable and had expedited surgery because of concerns about the severity of the AS, while the other eight had developed heart failure that prompted early surgery. Although recent guidelines6 still emphasise presence of symptoms as an important trigger for SAVR, heart failure (or any symptoms) in AS is an ‘end-stage’ manifestation with a dire outlook in a condition with a long asymptomatic phase. However, the tide may be about to turn, with recent evidence from randomised-controlled trials that early surgery is associated with markedly improved survival in asymptomatic patients with very severe7 or severe asymptomatic AS,8 when compared with conventional, guideline-driven practice. In light of these latest data, having patients develop heart failure after they have already been accepted for SAVR will become an untenable proposition.
Patients with AS not on the waiting list for SAVR
All seven such patients were under yearly cardiology follow-up with a diagnosis of ‘moderate aortic stenosis’, and three had been seen in a cardiology or valve clinic within three months of the index admission. The only difference we could identify was a longer total, pre- and postoperative hospital stay compared with patients who were on the waiting list (table 1). Patients in this group had numerically more severe indices of AS, and lower LVEF, suggesting more advanced disease, but the differences were not significant, presumably due to the low numbers involved. Clearly, the follow-up delivered by our valve clinics is imperfect, having failed to predict decompensation, albeit in a small number of patients. Potential reasons remain speculative due to the small size of the group, but may include underestimation of AS severity (although the higher gradients observed would identify these as cases of genuinely severe AS), reluctance of the valve clinic to refer for SAVR for clinical reasons (although the fact that patients ended up having SAVR mitigates against such a possibility) or under-reporting of symptoms by stoical patients. Whether a wider use of N-terminal proB-type naturietic peptide (NT-proBNP) levels and of aortic valve calcium scoring by computed tomography (CT)6 for monitoring AS progression can prevent such ‘near-misses’ remains to be seen.
Patients with AS not diagnosed until the index admission
The 10 patients in this category had a life-threatening complication of their valve disease as its first manifestation. No improvement in the efficiency of our current model of care for patients with AS would reduce this proportion; to achieve that, active screening for AS would be needed. In a previous study from the same geographic area, we found a prevalence of 8% of at least moderate mitral regurgitation (MR), and 5% of at least moderate AS (total prevalence 13%) in asymptomatic people over the age of 75,9 slightly higher than in the similar Ox-Valve study, which found a total population prevalence of moderate or severe valvular heart disease (VHD) of 11.3% in slightly younger subjects.10 The cost/benefit implications of such an approach need further study, but a primary care-based population-screening programme may turn out to be cost-effective and clinically feasible by potentially avoiding the long hospital stays associated with SAVR in patients with previously undetected AS.
Length of hospital stay (LOHS)
Consistently, patients with the least degree of planning of their SAVR (those with known AS but not on the waiting list, and those without a previous diagnosis of AS) spent longer in hospital than either those on the waiting list or those with a previous diagnosis of AS. The differences in the length of the pre-operative stay were most striking between patients on, versus those not on, the waiting list, with a mean of 2.2 ± 4.3 versus 20.0 ±11.8 days (p<0.001) (table 1), and were less marked for the postoperative stay. Patients without, versus those with, a diagnosis of AS had a mean pre-operative hospital stay of 22.3 ± 9.3 versus 6.0 ± 10.3 days (p<0.001), while the postoperative stay was not different. Our data are not granular enough to allow us to understand the exact causes of these differences, but they are likely to be a composite of delays relating to the availability of a bed to allow transfer from the admitting hospital to the surgical centre, and of the time it takes to stabilise and investigate acutely unwell patients.
Paradoxically, the finding of vastly longer LOHS in patients undergoing expedited or unplanned SAVR may be the most important motivator for improving the process of detection and monitoring of AS in the NHS. Further research is needed in order to model the net effect on healthcare costs of a screening programme for heart valve disease in the community.
Referral to treatment time (RTT)
The RTT for isolated SAVR in our hospital during the study period was 25 weeks. The significance of our findings is evidently dependent on the broader context of the healthcare system: patients who were already on the waiting list would not have had to present in extremis if the valve intervention could have been delivered sooner after diagnosis. Moreover, the goalposts are moving all the time, with the availability of transcatheter aortic valve replacement (TAVR) now reducing waiting list time to weeks after the diagnosis is made and the indication for valve intervention established.
Limitations
Size of the study
This is a small study, as it was designed and performed by busy clinicians rather than by dedicated research staff, and, as such, potentially significant differences between the various subgroups may not have reached the significance threshold. However, in spite of the low numbers, it offers an accurate ‘snapshot’ of the patient journey in a system with limited resources. We did not have the resources to collect detailed data on patients who had genuinely elective procedures, but our category of ‘expedited SAVR’ is likely to be broadly equivalent to those.
Assessment of myocardial damage
A limitation of our study is that imaging markers of myocardial damage, such as T1 mapping or late gadolinium enhancement (LGE) by cardiac magnetic resonance (CMR), were not available; such markers may allow further risk stratification of patients being followed-up for AS.
Relevance of SAVR in the TAVR era
The advent of percutaneous aortic valve replacement has changed the landscape of valve intervention beyond recognition,11 and the indications are expanding rapidly to encompass low-risk patients.12 It is likely that we shall see a further diminution of the proportion of patients undergoing SAVR, simultaneous to a major increase in the total number of aortic valve interventions, with open surgery reserved in the future for patients with concomitant pathologies, such as coronary artery disease not suitable for percutaneous coronary intervention (PCI) or aortic disease in need of surgical treatment. The proportion of patients for whom our findings are relevant will probably diminish, but SAVR is unlikely to become extinct, and the durability of TAVR valves is still not fully established, so our findings can still generate useful impetus for service improvement.
Conclusion
With the current model of care in the NHS, a significant proportion of patients have SAVR, the life-saving treatment for AS, performed in an unplanned manner, precipitated by clinical decompensation; such patients have a higher incidence of syncope. Unplanned surgery leads to prolonged hospital stay, an undesirable outcome. A small proportion of the unplanned operations are performed in patients without a prior diagnosis of AS; such patients could only be detected by rolling out a screening programme for heart valve disease in the community.
Key messages
- Intervening in cases of aortic stenosis requires a programme of close surveillance of the patients’ symptomatology, echocardiographic parameters and suitability for contemporary aortic valve replacement procedures
- While surgical aortic valve replacement in a suitable candidate offers unmatched results in terms of durability, performing this procedure in an expedited or unplanned fashion may lead to unnecessary morbidity in terms of length of hospital stay
- In a resource-limited public health programme, valvular screening programmes need to be enhanced to pick out cases of silent valvular degeneration and facilitate timely intervention of progressive aortic valve disease
Conflicts of interest
None declared.
Funding
None.
Study approval
Requirement for ethical approval and consent was waived by the local hospital audit department as the study is a retrospective analysis of anonymised patient data.
References
1. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. EuroIntervention 2022;17:e1126–e1196. https://doi.org/10.4244/EIJ-E-21-00009
2. Chambers JB. How to run a specialist valve clinic: the history, examination and exercise test. Echo Res Pract 2019;6:T23–T28. https://doi.org/10.1530/ERP-19-0003
3. Chambers JB, Parkin D, Rimington H et al. Specialist valve clinic in a cardiac centre: 10-year experience. Open Heart 2020;7:e001262. https://doi.org/10.1136/openhrt-2020-001262
4. Ross J. Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol 1985;4:811–26. https://doi.org/10.1016/S0735-1097(85)80418-6
5. Vollema EM, Amanullah MR, Ng ACT et al. Staging cardiac damage in patients with symptomatic aortic valve stenosis. J Am Coll Cardiol 2019;74:538–49. https://doi.org/10.1016/j.jacc.2019.05.048
6. Otto CM, Nishimura RA, Bonow RO et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35–e71. https://doi.org/10.1161/CIR.0000000000000932
7. Kang DH, Park SJ, Lee SA et al. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med 2020;382:111–19. https://doi.org/10.1056/NEJMoa1912846
8. Banovic M, Putnik S, Penicka M et al. Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis: the AVATAR trial. Circulation 2022;145:648–58. https://doi.org/10.1161/CIRCULATIONAHA.121.057639
9. Williams C, Mateescu A, Rees E et al. Point-of-care echocardiographic screening for left-sided valve heart disease: high yield and affordable cost in an elderly cohort recruited in primary practice. Echo Res Pract 2019;6:71–9. https://doi.org/10.1530/ERP-19-0011
10. d’Arcy JL, Coffey S, Loudon MA et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the Ox-VALVE population cohort study. Eur Heart J 2016;37:3515–22. https://doi.org/10.1093/eurheartj/ehw229
11. Arora S, Misenheimer J, Ramaraj R. Transcatheter aortic valve replacement: comprehensive review and present status. Tex Heart Inst J 2017;44:29–38. https://doi.org/10.14503/THIJ-16-5852
12. Spears J, Al-Saiegh Y, Goldberg D, Manthey S, Goldberg S. TAVR: a review of current practices and considerations in low-risk patients. J Interv Cardiol 2020;2020:2582938. https://doi.org/10.1155/2020/2582938