We aimed to describe the safety and tolerability of initiation of sodium-glucose cotransporter 2 inhibitors (SGLT2i) during hospitalisation with heart failure, and the frequency of, and reasons for, subsequent discontinuation.
In total, 934 patients who were not already prescribed a SGLT2i were hospitalised with heart failure, 77 (8%) were initiated on a SGLT2i a median of five (3–8.5) days after admission and two (0.5–5) days prior to discharge. During a median follow-up of 182 (124–250) days, SGLT2i were discontinued for 10 (13%) patients, most frequently due to deteriorating renal function. We observed reductions in body weight (mean difference 2.0 ± 0.48 kg, p<0.001), systolic blood pressure (mean difference 9.5 ± 1.9 kg, p<0.001) and small, non-significant reductions in estimated glomerular filtration rate (eGFR mean difference 2.0 ± 1.5 ml/min/1.73 m2, p=0.19) prior to initiation, with further modest reductions in weight (mean difference 1.2 ± 0.4 kg, p=0.006) but not systolic blood pressure (2.4 ± 1.5 mmHg, p=0.13) or eGFR following initiation of SGLT2i. At discharge the proportion prescribed a beta blocker (44% to 92%), angiotensin-receptor/neprilysin inhibitor (6% to 44%) and mineralocorticoid-receptor antagonist (35% to 85%) had increased.
In conclusion, inpatient initiation of SGLT2i was safe and well tolerated in a real-world cohort of patients hospitalised with worsening HF. We observed a 13% frequency of discontinuation or serious side effects.
Introduction

Sodium-glucose cotransporter-2 inhibitors (SGLT2i) improve symptoms,1 reduce hospitalisations and extend longevity2,3 for patients who have heart failure with reduced ejection fraction (HFrEF). These beneficial effects are observed very early following initiation,4 prompting calls for these agents to be given equal priority to more established therapies,5,6 which has been reflected in recent guidelines.7 Hospitalisation with heart failure (HF) offers the opportunity for optimisation of guideline-directed medical therapy (GDMT) including SGLT2i,8,9 however, the feasibility of doing so has not been reported outside of the artificial setting of randomised-controlled trials. In a real-world cohort of patients who were hospitalised with worsening HF, we aimed to first describe the safety and tolerability of initiation of SGLT2i in hospitalised patients, particularly in those concurrently receiving optimisation of other GDMT, and the frequency of and reasons for subsequent discontinuation.
Materials and method
Table 1. Baseline characteristics
| Demographics | |
|---|---|
| Mean age ± SD, years | 64.5 ± 14.0 |
| Male sex, n (%) | 59 (76.6) |
| Mean body mass ± SD, kg | 90.2 ± 25.2 |
| Aetiology, n (%) | |
| Ischaemic | 29 (37.7) |
| Non-ischaemic | 46 (59.7) |
| Unknown | 2 (2.6) |
| Past medical history, n (%) | |
| Ischaemic heart disease | 34 (44.2) |
| Hypertension | 34 (44.2) |
| Diabetes mellitus | 31 (40.3) |
| Atrial fibrillation | 32 (41.6) |
| Stroke/transient ischaemic attack | 10 (12.9) |
| Chronic kidney disease stage III/IV | 21 (27.3) |
| Chronic obstructive pulmonary disease | 15 (19.5) |
| Cardiac imaging, n (%) | |
| Mean LVEF ± SD, % | 25.4 ± 9.0 |
| Mean LVEDd ± SD, mm | 60.6 ± 7.2 |
| Right ventricular impairment, n (%) | 44 (57.1) |
| Regional wall motion abnormality, n (%) | 19 (24.7) |
| Medical therapy at admission, n (%) | |
| Beta blocker | 47 (61.0) |
| ACEi/ARB | 34 (44.2) |
| ARNI | 5 (6.5) |
| MRA | 27 (35.1) |
| Ivabradine | 4 (5.2) |
| Loop diuretic | 35 (45.5) |
| Antiplatelet | 26 (33.8) |
| Anticoagulant | 30 (39.0) |
| Key: ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin-receptor/neprolysin inhibitor; LVEDd = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; MRA = mineralocortoid-receptor antagonist; SD = standard deviation | |
Consecutive patients hospitalised for worsening HF between 1 November 2020 and 31 August 2021 at three teaching hospitals in the UK, who were initiated on SGLT2i prior to discharge were eligible for inclusion. Approval for the study was given following institutional governance review at each of the three hospitals. Data collection, storage and analysis were performed according to the Declaration of Helsinki and current General Data Protection Regulation (GDPR) guidelines. Patients were identified from clinical coding data and the records were reviewed manually to determine suitability for inclusion. We required patients to have had an unscheduled admission lasting ≥24 hours, in which the primary diagnosis was decompensated HF, and receipt of either significant augmentation of oral diuretics, intravenous diuretics or circulatory support. Patients with left ventricular ejection fraction (LVEF) ≥50% and those taking SGLT2i prior to admission were excluded. Patient demographics, past medical history and admission medications were recorded. The primary outcome was the frequency of and reasons for subsequent discontinuation of SGLT2i. Secondary outcomes were changes in body weight, systolic blood pressure and estimated glomerular filtration rate (eGFR), and changes in the provision and dosing of other GDMT.
Statistical analysis was performed using IBM SPSS Statistics version 26 (IBM Corporation, Armonk, NY). Normality of distribution was explored visually by distribution plots and confirmed using skewness tests. Continuous variables are presented as mean ± standard deviation (SD) if normally distributed, as median (interquartile range, IQR) if non-normally distributed, and discrete variables presented as number (percentage). Changes in renal function, blood pressure and body mass used paired t-tests. A p value <0.05 was regarded as statistically significant.
Results
During the study period 934 patients with LVEF <50% who were not already prescribed a SGLT2i were hospitalised for worsening HF. The median length of stay was eight (5–14) days, during which 77 (8%) patients were initiated on a SGLT2i prior to discharge. The mean age of patients initiated on SGLT2i was 64.5 ± 14 years, 59 (77%) were male, 74 (96%) had LVEF ≤40% and in 46 (60%) the aetiology was non-ischaemic cardiomyopathy (table 1).
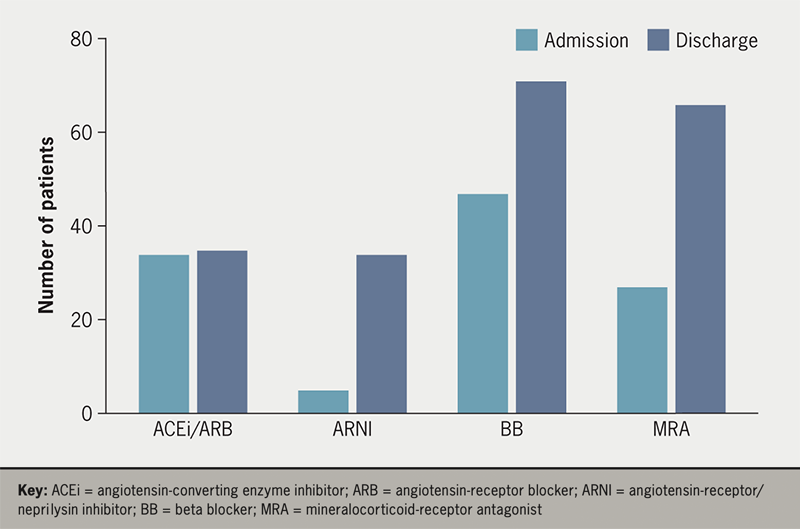
At the time of hospitalisation, 47 (61%) patients were receiving a beta blocker, 34 (44%) an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB), five (6%) were already taking an angiotensin-receptor/neprilysin inhibitor (ARNI), while 27 (35%) were prescribed a mineralocorticoid-receptor antagonist (MRA). At discharge the proportion receiving these agents had increased, with 71 (92%) prescribed beta blocker, 35 (45%) taking either ACEi or ARB, 34 (44%) receiving ARNI, and 66 (86%) prescribed MRA (figure 1).
During a median follow-up of 182 (124–250) days, SGLT2i were discontinued for 10 (13%) patients, most frequently due to deteriorating renal function (five), although only one patient had an eGFR <15 ml/min/1.73 m2. In two patients SGLT2i treatment was suspended within the first week of discharge without further measurement. One patient with type 2 diabetes mellitus who had discontinued their insulin therapy following symptoms of gastroenteritis, developed hyperglycaemic diabetic ketoacidosis (DKA) 18 days after initiation. Other reasons for discontinuation included hospitalisation with COVID-19 (one), administrative error (one) and appropriate withdrawal of medical therapy prior to death (two).
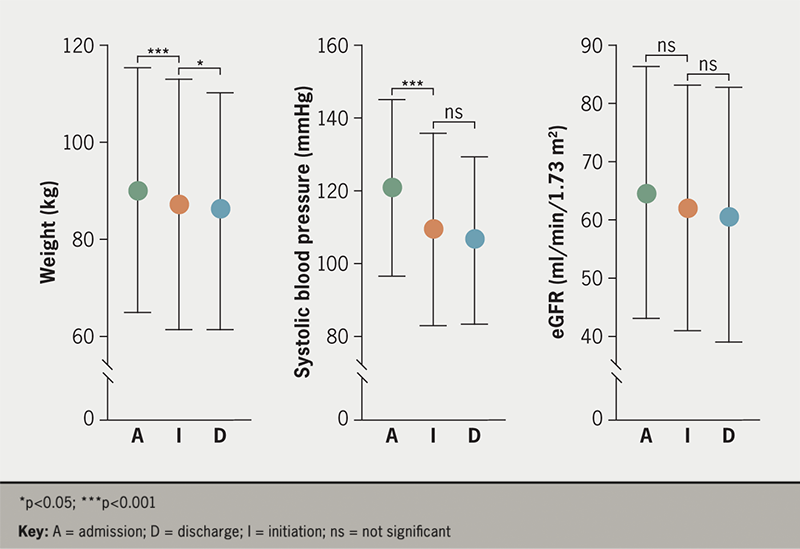
We observed reductions in body weight (mean difference 2.0 ± 0.48 kg, p<0.001), systolic blood pressure (mean difference 9.5 ± 1.9 kg, p<0.001) and small, non-significant reductions in eGFR (mean difference 2.0 ± 1.5 ml/min/1.73 m2, p=0.19) following admission, but prior to initiation of SGLT2i. Seventy-five patients (97%) received dapagliflozin and two (3%) empagliflozin. SGLT2i were commenced a median of five (3–8.5) days after admission and two (0.5–5) days prior to discharge. At the point of first receipt of SGLT2i, 54 (70%), seven (9%) and 16 (21%) were receiving oral, intravenous or no diuretics, respectively. Following initiation, we observed further modest reductions in weight (mean difference 1.2 ± 0.4 kg, p=0.006) but not systolic blood pressure (2.4 ± 1.5 mmHg, p=0.13) or eGFR (figure 2).
Discussion
Recent evidence suggests SGLT2i can be safely initiated during a hospitalisation with worsening HF,9 although their safety, tolerability and the frequency of discontinuation have not been reported beyond the setting of randomised-controlled trials. In the present study, we report outcomes in a real-world cohort of patients, demonstrating that SGLT2i initiation was safe and well tolerated, even where patients were receiving intravenous diuretics or concurrent optimisation of other GDMT. Initiation of SGLT2i was associated with further, modest reductions in weight, but not significant reductions in systolic blood pressure or renal function during admission. We acknowledge that diuretics, MRA and SGLT2i are likely to have contributed to the reduction in weight. However, no excessive diuresis was observed, which is reassuring given the early concerns about the use of SGLT2i in decompensated patients.
We observed a 13% frequency of discontinuation of SGLT2i following discharge from hospital. When this did occur, the most commonly documented reason was a deterioration in renal function, although this was often done so inappropriately. Given their established reno-protective benefits,2 and license in chronic kidney disease with eGFR ≥15 ml/min/1.73 m2,3 it is possible that alterations of diuretic dose or renin-angiotensin inhibitors would be preferable as an initial step, rather than the discontinuation of a class of medications with prognostic benefits. Existing data would support that SGLT2i cause a transient decline in eGFR on initiation, but that subsequent changes in renal function are more likely to represent other causes and should not prompt immediate cessation of the agent.10 Repeated measurement may in fact risk discontinuation of life-saving therapies, although we acknowledge renal function assessment is likely to be unavoidable following hospitalisation, or where the optimisation of renin-angiotensin inhibitors has been undertaken in parallel. One patient who had diabetes developed DKA, although this occurred during an intercurrent illness in which insulin was not administered and may not be directly attributable to SGLT2i therapy, but serves to remind clinicians of the importance of counselling patients of these risks.
Clinical inertia remains a major barrier to the optimisation of GDMT including the provision of SGLT2i for eligible patients. Hospitalisation for worsening HFrEF presents an opportunity for the optimisation of GDMT for those at the highest risk, who potentially have the most to gain. Despite this, our data show that the prevalence of SGLT2i initiation for potentially eligible patients remains low, even in specialist care settings. Clinicians caring for patients with heart failure can be reassured by our findings, which suggest that these agents are well tolerated, with a 13% prevalence of discontinuation or serious side effects, and do not seem to curtail the optimisation of other classes of GDMT.
Limitations
Our study includes a small number of patients who received SGLT2i at the discretion of their physician. Due to the retrospective nature of this study, inpatient data collection was performed by clinical judgement rather than predetermined time points, which may favour periods of increased clinical vulnerability regardless of proximity to admission, initiation of SGLT2i or discharge. The mean age of our study population is younger than a previous unselected cohort hospitalised with worsening heart failure from the same centre (mean 71 ± 14 years),11 and older patients may be less likely to receive optimised GDMT during hospitalisation. We do not report data for patients not commenced on SGLT2i during their admission, and these patients may have distinct clinical characteristics.
Conclusion
Our data serve to reassure clinicians that the inpatient initiation of these agents was safe and well tolerated in a real-world cohort of patients hospitalised with worsening heart failure. We observed a 13% frequency of subsequent discontinuation, and where this was done it was often done so inappropriately due to renal function.
Key messages
- For patients hospitalised with worsening heart failure, inpatient initiation of sodium-glucose cotransporter-2 inhibitors (SGLT2i) was feasible
- We observed a 13% frequency of discontinuation, which was usually due to renal function
- Initiation of SGLT2i during hospitalisation did not curtail the optimisation of other guideline-directed medical therapies
Conflicts of Interest
KKW has acted as an investigator and steering committee member for studies coordinated by Novartis, Cardiac Dimensions and Medtronic; has received speaker fees from Cardiac Dimensions, Medtronic, Microport, Abbott, Pfizer, Bayer and BMS; and has received unconditional research funding from Medtronic. JNG has received speaker fees from Medtronic, AstraZeneca, Novartis, Bayer and MSD; travel and hospitality from Bayer; and organised educational events supported by Novartis, Abbott, Boston Scientific and Medtronic. VKG has received speaker fees from AstraZeneca, Boehringer Ingelheim and Novartis. CG, HH, TAS, SS, TA, CC, MM, VN, JN: none declared.
Funding
This study was supported by a British Heart Foundation Clinical Research Training Fellowship awarded to SS (FS/CRTF/20/24071).
Study approval
Approval was given following institutional governance review (reference #9435, 18/06/2021) and, in view of the retrospective nature, individual patient consent was waived as appropriate data protection safeguards were in place. Data collection, storage and analysis were performed according to the Declaration of Helsinki and current GDPR guidelines.
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available due to the inclusion of potentially identifiable information but are available from the corresponding author upon reasonable request.
References
1. Kosiborod MN, Jhund PS, Docherty KF et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020;141:90–9. https://doi.org/10.1161/CIRCULATIONAHA.119.044138
2. Packer M, Anker SD, Butler J et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190
3. McMurray JJV, Docherty KF, Jhund PS. Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply. N Engl J Med 2020;382:973. https://doi.org/10.1056/NEJMc1917241
4. Berg DD, Jhund PS, Docherty KF et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2021;6:499–507. https://doi.org/10.1001/jamacardio.2020.7585
5. Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail 2021;23:882–94. https://doi.org/10.1002/ejhf.2149
6. Straw S, McGinlay M, Witte KK. Four pillars of heart failure: contemporary pharmacological therapy for heart failure with reduced ejection fraction. Open Heart 2021;8:e001585. https://doi.org/10.1136/openhrt-2021-001585
7. McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
8. Voors AA, Angermann CE, Teerlink JR et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–74. https://doi.org/10.1038/s41591-021-01659-1
9. Bhatt DL, Szarek M, Steg PG et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. https://doi.org/10.1056/NEJMoa2030183
10. Heerspink HJL, Cherney DZI. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol 2021;16:1278–80. https://doi.org/10.2215/CJN.02480221
11. Straw S, Byrom R, Gierula J et al. Predicting one-year mortality in heart failure using the ’Surprise Question’: a prospective pilot study. Eur J Heart Fail 2019;21:227–34. https://doi.org/10.1002/ejhf.1353
