Pulmonary hypertension is a rare disease, associated with a significant deterioration in quality of life, usually not curable and with an ominous prognosis. It is classified into five groups according to similar pathophysiological, clinical, haemodynamic, and treatment options in each of them. However, group 5 is the least common, encompassing different types of aetiological, semiological, and management conditions. We present the case of a patient diagnosed with precapillary component pulmonary hypertension, with the finding of an arteriovenous fistula at the peripheral level. Interventional exclusion was performed, achieving remission of her disease.
Introduction
Pulmonary hypertension (PH) affects less than 1% of the world’s population and is most commonly associated with normal or low cardiac output (CO), and only seldomly to high CO status. In the latter case, it is important to actively search for this aetiology, as in many cases its treatment can be curative. For example, large arteriovenous fistula (AVF) may lead to a state of high CO and consequently PH. Non-invasive imaging techniques are the cornerstone in the diagnostic algorithm, and endovascular treatment should be considered as first line. This case report reminds us that clinical suspicion should always be our starting point and that diseases with high mortality, such as PH, can be cured.
Case report
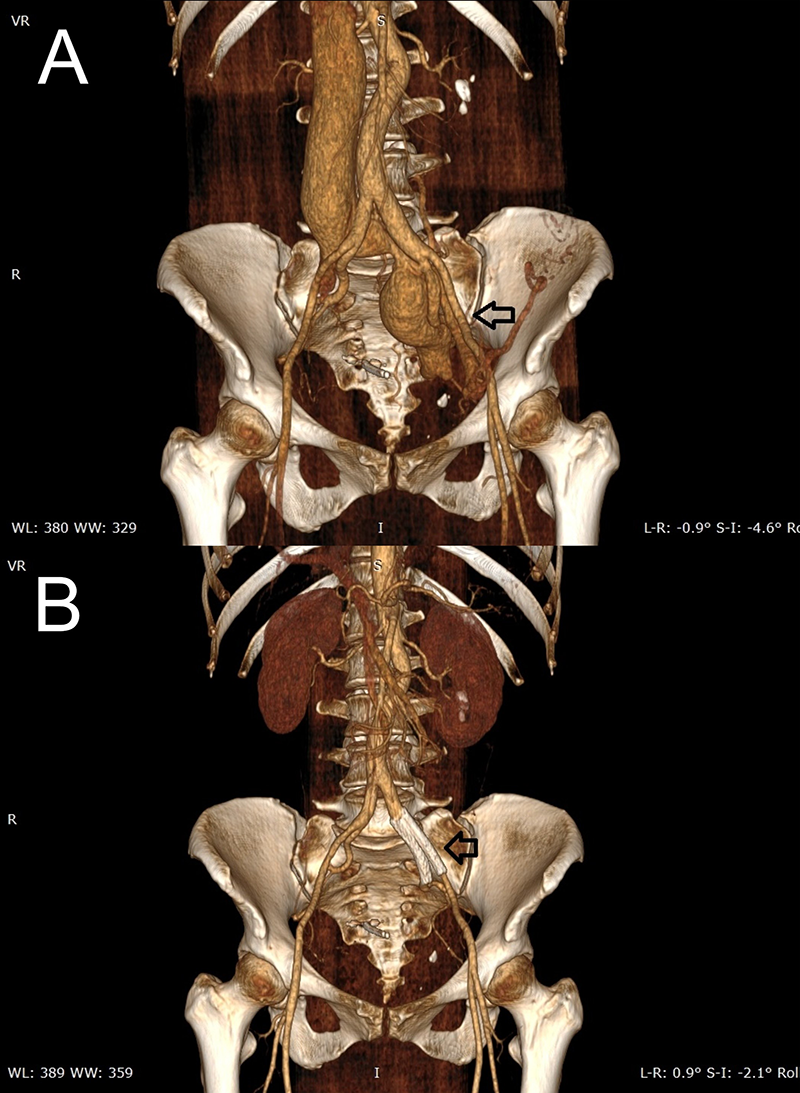
B. Previous AVF has been corrected after intravascular stent placement (black arrow)
A woman in her 40s presented to the emergency department with a two-year history of progressive dyspnoea and palpitations related to exertion. Important cardiovascular data included former tobacco smoking, hypertriglyceridaemia, and systemic arterial hypertension, currently under medical treatment.
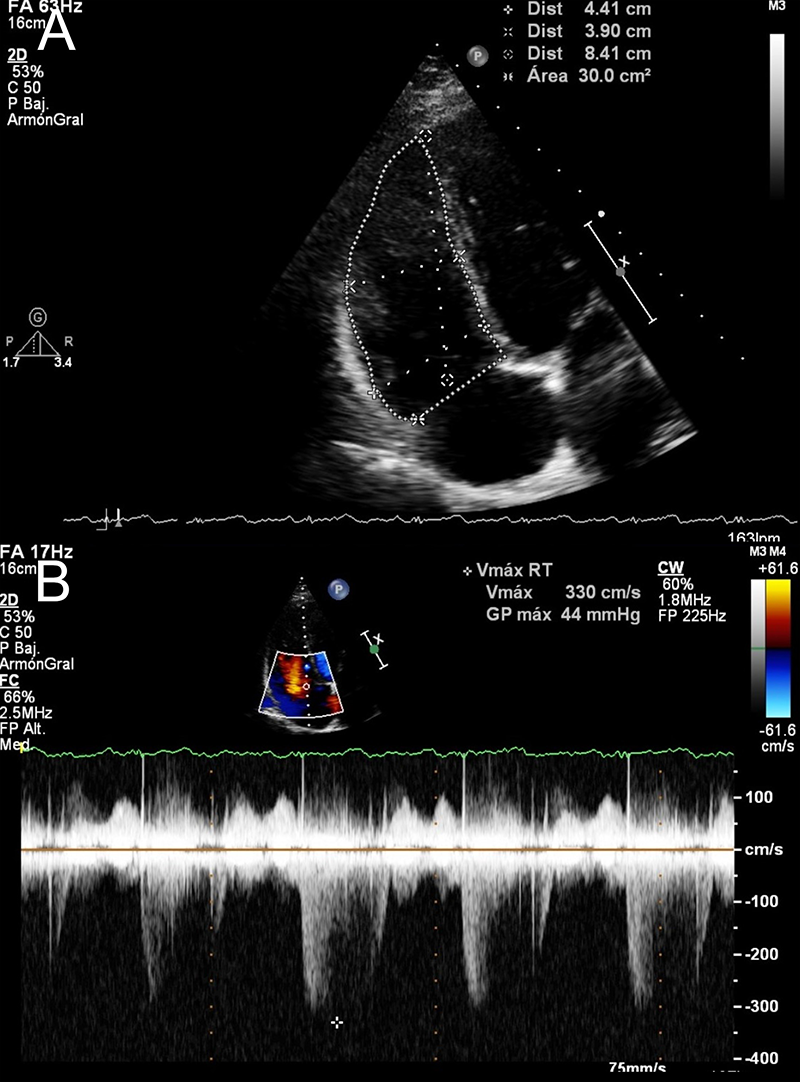
Physical examination revealed an intense and split S2 sound along with a holosystolic aortic murmur that radiated to the supra- and infra-aortic territories, mostly heard on the left abdominal flank. Routine blood tests, chest X-ray and electrocardiogram had no abnormalities. Echocardiogram revealed no left-side chambers alterations, along with dilatation of right ventricle (RV) and right atrium (RA) (figure 1A), and peak tricuspid regurgitation velocity (TRV) of 3.2 m/s (figure 1B), besides excluding any possible septal defect (shaken saline test). PH was suspected and additional tests ruled out both pulmonary parenchymal and thromboembolic disease. Computed tomography angiography (CTA) revealed an AFV communicating the external iliac left artery to the ipsilateral iliac vein, 7 mm wide with a post-confluence venous dilation 35 mm wide (figure 2A).
Right heart catheterisation (RHC) confirmed PH diagnosis, with a mean RA pressure of 4 mmHg, end-diastolic RV pressure of 5 mmHg, systolic pulmonary artery pressure (sPAP) of 45 mmHg, diastolic pulmonary artery pressure (dPAP) of 21 mmHg, mean pulmonary artery pressure (mPAP) of 31 mmHg, pulmonary artery wedge pressure (PAWP) of 1 mmHg and pulmonary vascular resistance (PVR) of 2.8 Wood Units. CO by thermodilution was 10.6 L/min (indexed at 6.35 ml/min/m2). Since frequent PH aetiologies were excluded, the patient’s case was classified as group 5 PH.
After consultation with the angiologist, AVF was completely excluded via intravascular closure and venous stenting. A first stent (53 × 50 mm) was placed at the proximal level of the external iliac artery, and the last one (50 × 45 mm) was placed in the proximal portion of the internal iliac artery (figure 2B). Six months later, a new RHC documented a RA media pressure of 0 mmHg, mPAP of 15 mmHg, and PAWP of 2 mmHg. CO was 6.3 L/min. Therefore, PH was considered to be cured and the patient was discharged to the angiology department.
Discussion
Despite its rarity, extensive research has been conducted in PH to improve patients’ quality of life and survival. The World Health Organization established the first classification of PH, which has evolved into the five categories recognised nowadays.1 The 2022 European Society of Cardiology (ESC) PH guidelines define PH according to haemodynamic criteria, with a mPAP >20 mmHg plus PVR >2 WU and PAWP <16 mmHg.2 Group 5 PH encompasses a heterogeneous range of conditions that may overlap with pulmonary vascular disease. Multiple factors (vasoconstriction, vascular remodelling, fibrotic changes) might contribute to the development of PH.1,2
Exercise and other physiological conditions may involve actual CO elevation, but characteristically with PVR decreasing. An imbalance between PVR, PAWP, and CO (especially when exceeding 8 L/min) must exist in order to lead to an increase in pulmonary pressure.3 High CO status might arise from congenital vascular malformations, as well as acquired ones like haemodialysis-AVF, known to be associated with PH in up to 30–60% of patients with end-stage renal disease (ESRD).4
AVF leads to volume overload with an increase in pulmonary flow and eventually to PH. Systemic artery angiography is the gold standard for diagnosis and treatment guidance, however, CTA is useful due to its non-invasive nature, reproducibility and satisfactory anatomic delimitation. A symptomatic AVF should be obliterated, either through surgical or endovascular technique. Gupta et al.5 described a PH case in an ESRD patient with AVF, with partial resolution after surgical exclusion. Shi et al.6 reported a case with PH diagnosis due to systemic-pulmonary AVF. Although the patient underwent definitive treatment, after one year there was no haemodynamic normalisation, unlike our case where remission was observed after six months. However, similar to ours, Brar and colleagues7 reported a 74-year-old patient with severe PH and heart failure and a congenital AVF between the renal artery and inferior vena cava. After endovascular percutaneous closure, normalisation of pulmonary pressure was achieved.
Key messages
- Pulmonary hypertension (PH) is a long-standing and incurable disease, however, when related to a correctable aetiology it is possible to provide definitive treatment, with a favourable evolution
- Although not frequently, both congenital and acquired arteriovenous fistula (AVF) might cause PH, due to a high cardiac output (CO) status and ultimately pulmonary vascular remodelling
- A thorough examination is essential to guide a proper diagnosis and therapeutic strategy
Conflicts of interest
None declared.
Funding
None.
Patient consent
We obtained patient’s consent in order to publish this article. Any information concerning the patient’s identity has been omitted.
References
1. Botswick AD, Melendres-Groves L. The story of group 5 pulmonary hypertension. Adv Pulm Hypertens 2021;20:40–5. https://doi.org/10.21693/1933-088X-20.2.40
2. Humbert M, Kovacs G, Hoeper MM et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J 2022;43:3618–731. https://doi.org/10.1093/eurheartj/ehac237
3. Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 2013;84:682–92. https://doi.org/10.1038/ki.2013.186
4. Qaiser KN, Sahay S, Tonelli AR. Pulmonary hypertension due to high cardiac output. Respir Med 2023;206:1–7. https://doi.org/10.1016/j.rmed.2022.107034
5. Gupta A, Farmer MJ, Farber H. A case of pulmonary hypertension associated with high cardiac output state from arteriovenous fistula or is it? Chest 2015;148(4 suppl):973A. https://doi.org/10.1378/chest.2281114
6. Shi YP, Li YD, Lv XZ, Yang YH. Systemic-pulmonary arteriovenous fistulae with pulmonary hypertension: a case report. Medicine (Baltimore) 2018;97:e9959. https://doi.org/10.1097/MD.0000000000009959
7. Brar V, Bernardo N, Suddath W, Weissman G, Asch F, Campia U. Reversal of pulmonary hypertension after percutaneous closure of congenital renal arteriovenous fistula in a 74-year-old woman. Cardiovasc Revasc Med 2015;16:310–12. https://doi.org/10.1016/j.carrev.2015.04.013
