This editorial series explores research in UK cardiology and acts as a practical guide to trainees interested in pursuing early career research. Two earlier entries in the series have explored the rationale for pursuing research and how to identify a project and supervisor. We now take a closer look at navigating the application processes, including funding opportunities and regulatory approvals, to get your research off the ground.
Introduction
This four-part editorial series aims to guide UK cardiology trainees and cardiovascular professionals through the key stages of early career research. Part 1 examined the role a period of research may play in a cardiologist’s career development,1 and part 2 explored how to identify the different research opportunities available.2 In this next part, we focus on the practical aspects of beginning your research journey; it explores the role of funders, sponsors, and regulatory bodies, as well as attempting to demystify the terminology associated with research logistics (online glossary).
Glossary. Common terms in the research application process
| What you may hear | What it means |
| BHF | British Heart Foundation. A UK-based charity that funds cardiovascular research including fellowships for healthcare professionals |
| CRTF/Fellowship | Clinical Research Training Fellowships. A funded programme lasting 2–3 years that provides a salary and research costs (with competitive application processes via number of funders) |
| GCP | Good Clinical Practice – a set of international standards for research. GCP training is provided by most institutions and valid certificates are needed for researchers. Keep yours in date and book a course early |
| HRA | Health Research Authority. The body that provides broad oversight of research within the UK to ensure compliance with regulatory standards. Submissions are made by IRAS. HRA approval is received after all the other steps |
| IRAS | Integrated Research Application system. An online submission portal for the regulatory approval of research in the UK. Requires a lot of careful form filling |
| JRMO | Joint Research Management Office – see JRO and research office. Naming difference in some institutions |
| JRO | Joint Research Office – see research office. ‘Joint’ as they provide integrated support between a research institution and NHS trust |
| NIHR | National Institute for Health Research. A body funded by the UK Department of Health and Social Care. Offers doctoral fellowships as well as charity and industry partnerships |
| MRC | Medical Research Council. A research council funded by the UK government that funds fellowships for healthcare professionals |
| OOPR | Out of programme for research. A structured process of taking up to 3 years out of a clinical training programme to pursue research. Liaise with your TPD and apply early |
| REC | Research Ethics Committee. Participant focused independent committees under the wider umbrella of the HRA. Following IRAS submission, discuss proposed research at a REC meeting before issuing an opinion to the HRA |
| Research Office | Research specialists based at the study sponsor. They will assist with regulatory approvals, study initiation and management. They are on your side and a great resource |
| Sponsor | The institution with oversight of the research. Normally the university to which your higher degree will be registered or the substantive employer of the research chief investigator. Will need to approve a research project once funded, but before regulatory approval |
| Wellcome Trust | A UK-based charity that funds PhD programmes. Submissions are made to individual programmes with differing remits and requirements |
Funding your time in research

By engaging with parts 1 (http://doi.org/10.5837/bjc.2023.027) and 2 (http://doi.org/10.5837/bjc.2024.011) of this series, you are likely now in a position to embark on a period of research; having identified both a supervisor and a project – a significant milestone in your journey. It is important to recognise the diversity in research types available, ranging from basic science and big data analysis (such as studies involving the UK Biobank) to clinical trials research. While the approval processes for these different types of research share similarities, they must be carefully adapted to suit the specific nature and requirements of the research being undertaken. The ensuing question then arises: what steps should you take next?
The research work itself, including your salary, equipment costs, and costs for the time of other staff members, will need to be covered. For most, this will entail a process of funding applications. Funding can come from various sources, including charities, government bodies, universities, and commercial industry. Navigating these different sources can be tricky, as each comes with its own benefits and disadvantages, and the application processes for each may differ substantially. These applications are perhaps the most daunting step in the early research process.
Funding bodies and fellowships
The British Heart Foundation (BHF), Medical Research Council (MRC), National Institute for Health and Care Research (NIHR), and the Wellcome Trust, provide significant funding to cardiovascular research in the UK, and play an important role in many clinician researchers’ careers through the provision of research fellowships. A research fellowship is a structured programme that provides funding and support for advanced training and research (clinical and basic science), with tailored versions often offered for healthcare professionals. These are generally two to three years in duration and designed to support obtaining a PhD or MD. The fellowship grant will generally include funding for your salary, research costs, university fees and (some) travel – though precisely what is offered will differ between funders. Getting a fellowship can be a tough, lengthy process and preparation is key. Certain projects may be better suited to a given funder and the application processes differ – as such, a frank discussion with your supervisor and research colleagues is highly advised, setting out clear expectations and timelines. It is a frequent occurrence to submit several applications or encounter initial rejections – such experiences should not lead to discouragement.
British Heart Foundation
The BHF is a UK charity and it is the largest independent funder of cardiovascular research.3 The BHF offers clinical research training fellowships (CRTFs) to early career clinical researchers (table 1). Applications are made online year-round and require a ‘case for support’ – a concise document detailing the specific research project, including experimental design and costings. Applications undergo external peer review, and the BHF fellowship committee then considers applications. There is no physical (or virtual) interview. The process may take up to six months for a single application.
Table 1. Common funders and fellowships
| Funder | Fellowship | Application | Costs covered | Considerations |
| BHF | CRTF13 | Online Open year round (4 panels per year) No interview Costed project required |
Salary Research costs (<£14,000/year) Tuition fees Travel (£500/year) |
2–3 years duration May resubmit amended unsuccessful applications Apply within 6 months of PhD registration |
| MRC | CRTF4 | Online 3 submission rounds per year (Jan, Apr, Sep) Interview Costed project required |
Salary Research costs including travel (<£25,000/year) Tuition fees |
Minimum 2 years May be unable to reapply if unsuccessful in the last 12 months or twice in total |
| Wellcome Trust | PhD fellowship programmes6 | Online Annual Applications made to individual programmes |
Salary Research costs including travel and equipment Tuition fees Carbon offset costs |
3 years Remits and requirements of programmes will differ widely |
| NIHR | Doctoral fellowships14 | Online Biannual (Apr and Oct starts) |
Salary Research costs Tuition fees |
3 years Industry and charity fellowships advertised online15 |
| Heart Research UK | PhD Studentship8 | Online Annual (Jun) Costed project required |
Salary Research costs (up to £7,500/year) Tuition fees |
3 years Salary for clinical studentships is co-funded with participating host institutions |
| Key: BHF = British Heart Foundation; CRTF = clinical research training fellowship; MRC = Medical Research Council; NIHR = National Institute of Health Research | ||||
Medical Research Council
The MRC, one of the seven councils comprising ‘UK Research and Innovation’ (UKRI), is funded by the UK government. It provides CRTFs and other funded research opportunities to medical professionals.4 Applications are made online with rounds closing every January, April and September. Applications should align with the MRC’s remit;5 this includes global health, molecular and cellular medicine, population and systems medicine and translational research. These applications are independently assessed, reviewed by a research subcommittee, and short-listed applications are then invited for a 30-minute interview. This process takes around six months.
Wellcome Trust
The Wellcome Trust is a UK-based charity that funds PhD fellowships for healthcare professionals.6 These are separated into specific programmes that recruit annually and work only with named institutions. It is important to review the individual programmes and assess how your research interests may align – there may not be any programmes that are suitable. Current programmes include those focused on global health research in Africa, health priorities in the global south and health advances in underrepresented groups. Applications are made directly to the individual programme. Unlike the BHF and MRC CRTFs, a specific costed project is usually not required. Following review, shortlisted candidates are invited for a panel interview prior to a decision being made.
National Institute of Health Research
The NIHR is funded by the UK Department of Health and Social Care with a focus on early translational, clinical, and applied health and social care research within the NHS.7 The NIHR offers doctoral fellowships, as well as fellowship programmes in partnership with charities and industry. Applications are made online and shortlisted applicants are interviewed. Programmes commence in April and October each year.
Industry funding
Commercial businesses within the healthcare industry, such as large pharmaceutical companies, may offer full or partial funding for research – in collaboration with research councils and academic institutions. An industry partnership can benefit from state-of-the-art equipment, increased financial support, cross-functional training, and networking opportunities. Industry-funded PhDs often focus on applied research, which can limit the scope of research opportunities. There may also be concerns over conflicts of interest and publishing restrictions, especially when there are commercial implications. Industry-funded projects are often advertised online or exist through the connections of a research team – so speak to your supervisor about potential opportunities.
Other institutions and short-funding opportunities
Smaller charities and those associated with NHS trusts may also offer funding.8 These may not cover a full PhD programme but can fund a proportion of the costs or fund one to two years from which further applications can then be made. These are worth exploring with your supervisor and local research teams.
Other important considerations
For cardiology trainees a fellowship salary will be provided commensurate to your level of training (for most funders including the BHF, MRC and the Wellcome Trust). The timing of research within training was explored in an earlier part of this series,1 but this is worth thinking about at this stage – largely as the increase in pay at ST6 is significant.
Going ‘out of programme’
This section is focused on UK cardiology trainees with a national training number. Structured processes exist to allow trainees to temporarily step out of their clinical training programme. One form of this ‘out of programme for clinical research’ (commonly referred to as OOPR) is another important logistical step in the research process for trainees. Here, we explore how to navigate this process and some common pitfalls along the way.
OOPR
OOPR allows trainees to undertake research, normally for a higher degree, and later return to their clinical training. On return, trainees are guaranteed a clinical training post within their region, but not a specific post. The duration of OOPR is normally three years (with a four-year maximum in exceptional circumstances) and applications are managed regionally. Applications should be submitted at least six months prior to the intended start date and details of the research project, expected OOPR duration, and how it will contribute to career development will be required. There will be a degree of local variation in this process so discussion with your educational supervisor, project supervisors, and training programme director to set expectations and clarify the full requirements is important. OOPR approval is not automatic but with planning should not be an obstacle.
While OOPR you will be employed and managed by the institution through which you are funded, but you will still be invited to an academic annual review of competence progression (ARCP). This review will include the submission of a named academic supervisor’s report. As you progress through the OOPR you should keep in touch with your clinical training programme director (TPD), especially with regards to return to clinical training.
Sponsors
A sponsor can be an institution like an NHS trust, a university, or a private company. The sponsor is responsible for the initiation, management, indemnity, and overview of financing for research. In simple terms, they provide assurance to regulatory bodies and funders that your research is feasible, legal, and being carried out safely. They provide support in obtaining regulatory approvals and in the ongoing monitoring of a study. For a cardiology trainee undertaking a research fellowship this will generally be the university to which your studies are registered. Otherwise, it will be the substantive employer of the chief investigator of the research. Universities will have a research office (sometimes also known as a joint research management office or JRMO) that you will work closely with.
Sponsor approval
With funding approved, the sponsor’s research office will need to formally review and approve the proposed research. Until sponsor approval has been received it is not possible to apply for further regulatory approval. The precise nature of this process will be governed by local policies, but, in general, this will require the submission of a detailed study pack including the protocol, costings, and study-specific documents.9 This process is very variable in length but is likely to take at least four to six weeks.
Regulatory approvals
Research in the UK, encompassing various types, necessitates regulatory approvals, which are facilitated through a shared online system – the Integrated Research Application System (IRAS). While these approvals are pertinent across different research domains, the forthcoming discussion will be most relevant to those undertaking clinical research, particularly within the NHS context. However, the need for different regulatory bodies depending on the nature of the research is summarised in table 2. Your study sponsor will play a crucial role in guiding you through this process, but a clear understanding of the key terms and procedures is invaluable.
Table 2. Common regulatory approvals
| Requirement | Basic science research | Big data research | Clinical trials research |
| Ethics approval | Required, focuses on lab practices, animal use (if applicable), and data handling | Required, emphasises data privacy, consent, and ethical use of data | Required, stringent due to direct patient involvement, safety, and consent |
| Funding approval | Depends on the source (e.g. grants, institutions); often project specific | Often needed for data access, computational resources; varies by funding source | Critical, especially for trials; includes funding for patient care, data management, etc. |
| Data protection | Necessary for handling research data; adherence to data protection laws | Paramount, especially with large datasets and personal information | Essential for patient data; strict compliance with data protection laws |
| Institutional (site) review | Institutional review boards assess research protocols, safety, etc. | Institutional review for data handling, ethical implications | Institutional review boards focus on patient safety, protocol adherence |
| Health research authority approval | May be required for studies involving NHS data | Required if using NHS data; focuses on data governance | Essential for studies involving NHS patients or data |
| MHRA approval | Not applicable | Not applicable | Required for trials with medicinal products or devices; assesses safety and efficacy |
| Clinical trials authorisation | Not applicable | Not applicable | Required; a regulatory approval for the trial protocol by MHRA |
| NHS permissions | If using NHS facilities or data | Required for accessing NHS data or patient cohorts | Necessary for trials within NHS settings; involves permissions for patient involvement |
| Confidentiality advisory group approval | If accessing confidential patient data without consent | Often required for accessing confidential patient data without consent | May be needed for accessing patient data without explicit consent |
| Human tissue authority licence | If research involves human tissue | If research involves storing/using human tissue | Required if the trial involves the use of human tissue |
| Key: MHRA = Medicines and Healthcare products Regulatory Agency; NHS = National Health Service | |||
Health Research Authority
The Health Research Authority (HRA) oversees the regulation of all research involving NHS patients and staff, as well as participants, data, or tissue from health and social care settings. HRA provides broad regulatory oversight; ensuring that research complies with legal, ethical, and governance standards. HRA approval is the last thing that will come into place for any clinical research within the NHS. This will normally take around two to three months from application, but may be longer.
Research Ethics Committees
Research Ethics Committees (RECs) are independent groups that review research proposals involving human participants – to safeguard their rights, safety, dignity, and well-being. They sit within the HRA and feedback their opinion – so HRA approval will not be received without REC approval first.
IRAS
The Integrated Research Application System (IRAS) is an online system used in the UK to facilitate the application process for permissions and approvals of health research, acting as a point of contact with the HRA and RECs.10 Submissions involve completing a detailed online form summarising the proposed research and uploading all current study documents. The precise information required will differ depending on the type of research being proposed. It is important that this is consistent with information submitted to the sponsor and any study documents. Final submission will require sign off from the study sponsor. Once submitted, a HRA approvals specialist will review the proposal and may seek clarification.
REC meeting
Once an IRAS submission has been made, a REC meeting date can be booked (also using an online portal). These are now generally virtual and the REC itself does not have to be local to your study site or sponsor. There may be a significant interval until the next available REC meeting date. The meeting will involve discussing the proposed research with a panel including lay members of the public. After this, the REC will issue an opinion: favourable, favourable with conditions (which will need to be addressed before approval is granted) or unfavourable.
Getting off to a good start
With funding, OOPR, sponsor and regulatory approval in place, a research project can (finally) start. To make this start as smooth as possible there are a few a simple things that can help (and can be completed during any of the many periods of waiting):
- Good clinical practice (GCP) training – GCP is a set of internationally recognised ethical and scientific quality requirements that must be followed in clinical trials. Training courses last one to two days and should be refreshed every two to three years. Having a certificate ready and up to date will save any unplanned delays. Check your sponsor for course dates in advance.
- Check the processes for local study approval. This will depend on your specific research and intended study sites. The sponsor and any study site research offices will be able to advise.
- Discuss with your supervisor and research team if they envisage a certain statistical package or programming language will be used. Try to learn some basics and enrol on courses in advance – basic statistical training at least is always valuable.
- If your research will involve working with animals – apply for the appropriate Home Office licences using the Animals in Science Procedures e-Licensing (ASPeL) system.11 You will need a personal and project licence at least.
Personal experiences
Check out the OOPR resources on the BJCA website for more advice and to hear first-hand stories from cardiology trainees who have been through the process.12
Conclusion
The research application process is lengthy and requires a planned, thoughtful approach to best tackle its multiple steps, but, fortunately, cardiology trainees in the UK have access to excellent opportunities and support (figures 1 and 2). At an early stage, speak with your supervisor about appropriate funding applications and liaise with your TPD about OOPR. As these come into place, engage with your sponsor’s research office to obtain regulatory approvals, and ultimately get started on your research.
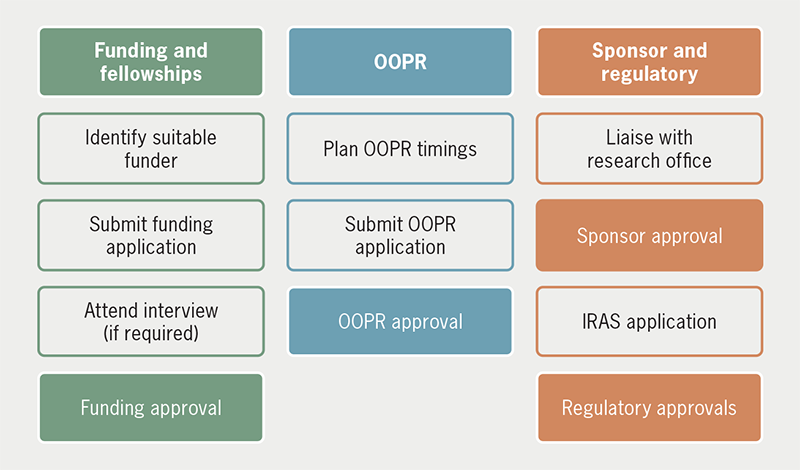
| Key: IRAS = Integrated Research Application System; OOPR = out of programme for clinical research |
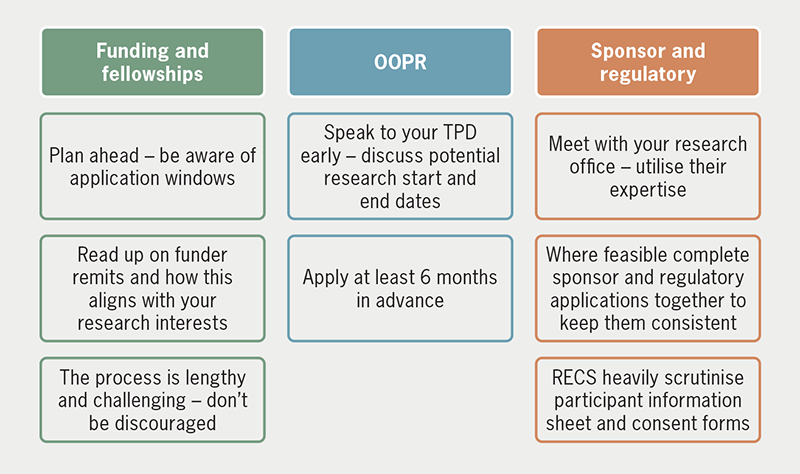
| Key: OOPR = out of programme for clinical research; RECS = research ethics committees; TPD = training programme director |
Key messages
- The entire application process, from writing a funding application to receiving final regulatory approvals, can take over 12 months (even if all goes well). Think ahead and map out the likely timings at the start
- The process of successfully applying for funding alone will take over six months. Discuss the most appropriate applications with your supervisor and read their requirements and processes in advance
- Speak to your training programme director (TPD) about your research plans early. Submit an out of programme for clinical research (OOPR) application at least six months in advance of any proposed research start date
- Utilise the expertise of your sponsor and engage with the specialists in the research office to aid with all these steps, and, importantly, the applications for regulatory approvals
- Supervisors and peers have faced this before and can help. Don’t try to go alone and know that setbacks are common but not insurmountable
Conflicts of interest
None declared.
Funding
None.
Editors’ note
This is the third part of a series of articles on Research in Cardiology.
Part 1 is available at: http://doi.org/10.5837/bjc.2023.027.
Part 2 is available at: http://doi.org/10.5837/bjc.2024.011.
References
1. Kurdi H, Abiodun A, Westwood M, Camm CF. Navigating the research landscape in cardiology. Part 1: research – career necessity or bonus? Br J Cardiol 2023;30:91–4. https://doi.org/10.5837/bjc.2023.027
2. Kurdi H, Artico J, Lodge F, Camm CF. Navigating the research landscape in cardiology. Part 2: finding the right research. Br J Cardiol 2024;31:32–5. https://doi.org/10.5837/bjc.2024.011
3. British Heart Foundation. What we do. Available at: https://www.bhf.org.uk/what-we-do [accessed 26 November 2023].
4. UK Research and Innovation. Predoctoral clinical research training fellowship. Available at: https://www.ukri.org/opportunity/pre-doctoral-clinical-research-training-fellowship/ [accessed 26 November 2023].
5. UK Research and Innovation. Medical Research Council. Remit, programmes and priorities. Available at: https://www.ukri.org/councils/mrc/remit-programmes-and-priorities/ [accessed 7 December 2023].
6. Wellcome. PhD Fellowships for Health Professionals. Available at: https://wellcome.org/grant-funding/schemes/phd-fellowships-health-professionals [accessed 26 November 2023].
7. National Institute for Health and Care Research. Who we are. Available at: https://www.nihr.ac.uk/about-us/who-we-are/ [accessed 29 November 2023].
8. Heart Research UK. Apply for a PhD studentship. Available at: https://heartresearch.org.uk/phd-studentship/ [accessed 26 November 2023].
9. Joint Research Management Office. Setting up a study. Available at: http://www.jrmo.org.uk/performing-research/conducting-research-in-the-nhs/setting-up-a-study/ [accessed 26 November 2023].
10. Integrated Research Application System. Homepage. Available at: https://www.myresearchproject.org.uk/ [accessed 7 December 2023].
11. UK Government. Animal testing and research: guidance for the regulated community. Available at: https://www.gov.uk/guidance/research-and-testing-using-animals#apply-for-licences [accessed 29 November 2023].
12. British Junior Cardiologists’ Association. OOPR Resources. Available at: https://bjca.tv/oopr-resources/ [accessed 7 December 2023].
13. British Heart Foundation. Clinical Research Training Fellowships. Available at: https://www.bhf.org.uk/for-professionals/information-for-researchers/what-we-fund/clinical-research-training-fellowships [accessed 7 December 2023].
14. National Institute for Health and Care Research. NIHR fellowship programme. Available at: https://www.nihr.ac.uk/explore-nihr/academy-programmes/fellowship-programme.htm [accessed 7 December 2023].
15. National Institute for Health and Care Research. Funding opportunities. Available at: https://www.nihr.ac.uk/researchers/funding-opportunities/?custom_in_type=11666 [accessed 7 December 2023].
