Large-scale observational data have demonstrated a robust, independent association of elevated lipoprotein(a) (Lp[a]) levels with atherosclerotic cardiovascular disease (CVD), stroke, and peripheral artery disease. Elevated Lp(a) concentrations in patients with ischaemic heart disease have been linked to higher rates of peri-procedural myocardial infarction (MI) during percutaneous coronary intervention (PCI). This association could be attributed to the proatherogenic, proinflammatory, and potentially antifibrinolytic properties of the Lp(a) particle. In addition, there is an increased prevalence of high-risk plaque features, such as heavy macrophage infiltration, large necrotic lipid core, and thin-cap fibroatheromas in patients with elevated Lp(a) concentrations presenting with acute coronary syndrome. Despite growing evidence linking elevated Lp(a) to incident CVD, its association with recurrent ischaemic cardiovascular (CV) events has been less clear. Multiple observational studies have assessed the effect of elevated Lp(a) levels on major adverse cardiovascular events (MACE) and rates of restenosis in patients with previous PCI. This review summarises the available data on the modulatory effect of Lp(a) on plaque morphology and recurrent ischaemic events.
Introduction
Lipoprotein(a) (figure 1) has emerged as an independent and causal risk factor for cardiovascular diseases (CVDs) and cerebrovascular diseases with prospective epidemiological studies demonstrating a link between elevated Lp(a) levels and increased incidence of ischaemic heart disease (IHD) and myocardial infarction (MI).1 The value of this association is that it was seen to be independent of traditional cardiovascular (CV) risk factors including diabetes, hypertension and smoking.1 Moreover, Lp(a) levels greater than 50 mg/dL were associated with a threefold increase in acute coronary syndrome (ACS) in younger patient cohorts (age <45 years).2
![Zaman - Figure 1. Lipoprotein(a) (Lp[a]) particle containing apolipoprotein B100 and apolipoprotein(a)](https://bjcardio.co.uk/wp-content/uploads/2024/07/Zaman-Figure-1.png)
| Key: KIV: kringle IV; KV: kringle V; Lp(a): lipoprotein(a) |
The exact physiological role of Lp(a) remains not fully understood. One suggestion is that the molecule plays a role in tissue repair and wound healing; however, individuals with no measurable Lp(a) level manifest no identifiable disease. The pathological role of Lp(a) is linked to atherosclerosis through the lipoprotein moiety, and thrombosis through the plasminogen-like apolipoprotein(a) moiety.3 Lp(a) can accumulate within the intima of arteries and on the aortic valve leaflets.4 Lp(a) promotes the formation of atherosclerotic plaques via several mechanisms. Multiple studies have indicated that Lp(a) is taken up by macrophages to produce foam cells in a process that promotes the development of atherosclerotic lesions.4 It induces expression of inflammatory cytokines and tumour necrosis factor and increases expression of adhesion molecules on the surface of endothelial cells, thus promoting endothelial damage and dysfunction. Additionally, Lp(a) binds and transports circulating oxidised phospholipids which are involved in plaque vulnerability.4 Oxidised phospholipids also mediate arterial wall inflammation and promote monocyte inflammatory response.4 Due to the complex morphology of atherosclerotic plaques in patients with high Lp(a) levels, coronary atherosclerosis in this patient cohort usually manifests clinically as MI rather than stable angina.5
The homology to plasminogen drives thrombogenicity of Lp(a) by competing with plasminogen for binding sites on endothelial cells, which in turn inhibits fibrinolysis, thereby promoting thrombosis. Lp(a) also inhibits tissue plasminogen activator (t-PA) binding to fibrin, and therefore prevents plasminogen activation by t-PA. Some studies have suggested that apolipoprotein(a) attenuates t-PA-mediated plasminogen activation by direct competition of apolipoprotein(a) with plasminogen for binding sites on fibrin’s surface.6 It is possible that Lp(a) promotes thrombosis by promoting coagulation.
ACS and vulnerable plaques
ACS occurs as a result of a plaque erosion or rupture together with a formation of superimposed thrombus restricting coronary flow. Intravascular imaging with the use of high-resolution spatial optical coherence tomography (OCT) has shown that patients with high Lp(a) levels exhibit high-risk features, including plaques with a high lipid burden, large necrotic core and thin-cap fibroatheromas in vulnerable coronary plaques.5 Clinical studies have prospectively assessed plaque morphology by OCT in patients with ACS and elevated Lp(a) levels with high Lp(a) groups defined as an Lp(a) level ≥30 mg/dL. These studies examined plaque characteristics at the site of culprit stenosis and confirmed that in the cohort of patients with high Lp(a) levels, there was a higher prevalence of vulnerable plaque features – lipid-rich plaques, thin-cap fibroatheromas, and wider lipid arcs.7 A subsequent retrospective study also compared OCT findings in elevated Lp(a) and low Lp(a) level groups. The threshold for a high Lp(a) level in this study was defined as an Lp(a) level >25 mg/dL. The study included 255 patients and confirmed a higher prevalence of lipid-rich plaques in the culprit lesion in the high Lp(a) level group with thinner fibrous caps.8 A post hoc analysis of six randomised clinical trials using intravascular ultrasound (IVUS) assessment of coronary atheromas in patients with elevated Lp(a) levels stratified patients into high Lp(a) (>60 mg/dL) and low Lp(a) (<60 mg/dL) level groups. The baseline characteristics were well matched and most patients in both groups (>95%) received statin therapy. Following adjustment for multiple co-variates, IVUS analysis demonstrated increased volumes of coronary atheromas in the high Lp(a) level group compared to the low Lp(a) level group.9
Case study
A 48-year-old male, with no previous medical history, was admitted with intermittent symptoms consistent with ischaemic-type chest discomfort, with the longest episode lasting approximately one hour. He had no conventional risk factors for IHD and was active and exercised regularly. His height was 185 cm and weight 75 kg, with a body mass index (BMI) of 22 kg/m2. The admission electrocardiogram showed biphasic and deeply inverted T-waves in the anterior leads, consistent with left anterior descending artery (LAD) ischaemia (figure 2). His troponin peaked at 13,323 ng/L and other biochemical blood results were unremarkable demonstrating normal renal and liver function tests; HBA1c was 35 mmol/mol; total cholesterol, 5.6 mmol/L; triglycerides, 1.30 mmol/L. His admission Lp(a) concentration was elevated at 202 nmol/L – a value identified by the UK consensus statement on Lp(a) to be high risk for CV events.10 An echocardiogram showed preserved biventricular systolic function (>55%) without valvular abnormalities.
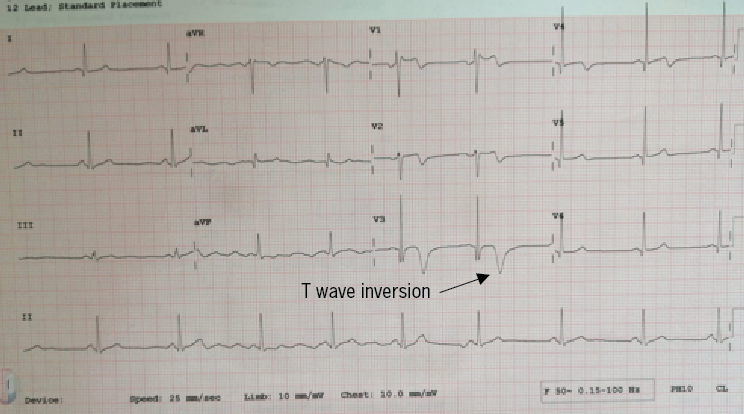
The coronary angiogram demonstrated severe mid-LAD stenosis just distal to a bifurcation with the diagonal branch (medina 0,1,1), a further severe lesion was seen mid-vessel, and mild atheromas were seen in the remainder of the coronary tree (figure 3). An OCT demonstrated diffuse lipid-rich plaque, necrotic core and a wide lipid arc of 270 degrees. Minimal luminal area in the mid-LAD measured 2.2 mm2. Plaque erosion was seen in the mid-LAD with an adherent platelet- and fibrin-rich white thrombus (figure 4). The mid-LAD stenosis was provisionally treated with implantation of a single drug-eluting stent (DES) with a good angiographic and OCT result.
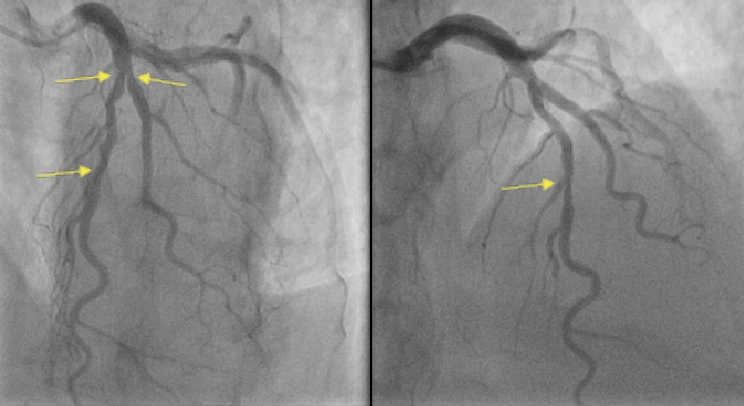
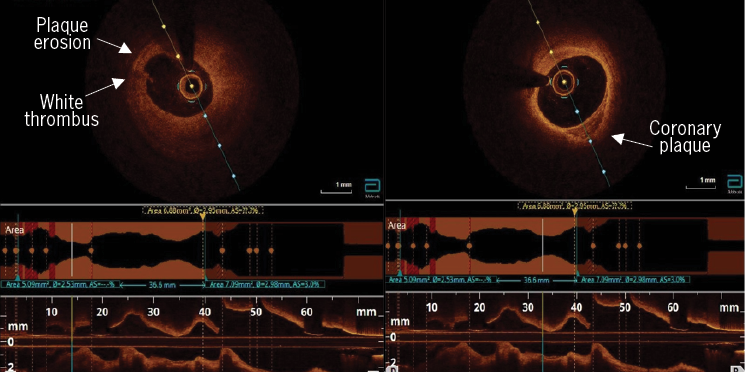
This case further supports the findings that are consistent with available study data on the assessment of plaque morphology by OCT, IVUS and computed tomography coronary angiogram (CTCA) in patients with elevated Lp(a) levels. In this case, diffuse areas of atherosclerosis with high-risk plaque features were seen in a young, fit and otherwise well patient presenting with ACS without any conventional risk factors for coronary artery disease (CAD). Similar findings were reported in the OCT and IVUS studies outlined above. A recently published ad hoc analysis from the DIAMOND trial, which assessed plaque morphology with CTCA in patients with Lp(a) levels >70 mg/dL, identified accelerated progression of low-attenuation coronary plaque volume.11 The DIAMOND trial was a prospective randomised trial that investigated the impact of dual antiplatelet therapy on coronary atherosclerotic disease progression with a predefined subgroup analysis of patients with measured Lp(a) levels who underwent serial CT coronary angiography 12 months apart. Patients with Lp(a) concentrations >70 mg/dL exhibited accelerated progression of high-risk plaque features despite receiving guideline-directed medical therapy.
Secondary prevention therapy – impact on Lp(a) levels
The majority of data linking elevated Lp(a) concentrations to incidence of IHD is based on studies in the general population. Overall, the role of elevated Lp(a) concentrations in secondary prevention has been less clear given the use of optimal medical therapy for this patient cohort. Prior data suggesting that the role of aspirin in the management of patients with elevated Lp(a) is controversial. Primary prevention with aspirin trials have not shown benefit due to increased bleeding events;12 however, data extrapolation from a retrospective analysis of the Women’s Health Study suggested that patients with elevated Lp(a) levels benefited from aspirin therapy with a twofold CV risk reduction.13
The use of statin therapy for the management of patients with elevated Lp(a) levels has also been extensively studied. Overall, the data show conflicting results with some studies suggesting a positive therapeutic effect of statin therapy on Lp(a) reduction. A recently published meta-analysis, however, concluded that statins did not lead to clinically important reductions in Lp(a) levels when compared to placebo in patients at risk of CVD.14
Recurrent ischaemic events
Given the controversial results of established CV therapies on Lp(a) reduction, some studies assessed the prevalence of recurrent ischaemic events in patients with elevated Lp(a). One registry prospectively enrolled over 12,000 consecutive, unselected patients to assess the outcomes of percutaneous coronary intervention (PCI). Patients were divided into a high Lp(a) group with Lp(a) levels >30 mg/dL, and a low Lp(a) group with Lp(a) levels <30 mg/dL. Baseline characteristics, including conventional CV risk factor profiles, were similar across both groups. Findings revealed that more patients in the high Lp(a) group had multi-vessel disease (60.3% vs. 56.6%). At a median follow-up of 7.4 years, primary outcomes, including CV death, MI and ischaemic stroke were significantly increased in the high Lp(a) group. Rates of repeat revascularisation, including target-vessel and target-lesion revascularisation, were also increased in the high Lp(a) group.15 Contrary to the above, the ad hoc analysis of the randomised dal-OUTCOMES clinical trial identified no difference in recurrent ischaemic events in patients with elevated Lp(a) concentrations who were established on appropriate secondary prevention therapy. The median Lp(a) concentration was 12.9 mg/dL in study patients and 12.1 mg/dL in control patients.16 Similar results were observed in a meta-analysis of the PEACE, CARE and PROVE IT-TIMI 22 trials. In these trials, higher Lp(a) concentration was not consistently associated with traditional risk factors, except slightly higher levels of LDL cholesterol and apolipoprotein B. Furthermore, this meta-analysis found no association between Lp(a) concentrations and prediction of future MACE. However, when the results of this meta-analysis are combined with those from other similar secondary prevention trials, increased Lp(a) concentration was found to be significantly associated with the risk of CV events in patients with established CAD.17 This association was demonstrated with Lp(a) levels >49 mg/dL. Having said that, marked heterogeneity was seen across the trials. Some Lp(a) observational studies reported increased risk of subsequent MACE only in patients with pronounced elevation of serum LDL cholesterol. Those findings are in contrast to a study from China, which enrolled patients with LDL <1.8 mmol/L and observed an increased rate of unplanned revascularisations at one-year follow-up in the high Lp(a) group.18
In-stent restenosis
In-stent restenosis (ISR) remains a major drawback of PCI due to biological-, mechanical-, patient- and operator-related factors, and other mechanisms with the prevalence of ISR thought to be as high as 10% of all PCIs. It is also suggested that Lp(a) may accumulate within the walls of an injured vessel following stent implantation and induce proliferation of smooth muscle cells leading to neo-atherosclerosis.19 Multiple studies have identified the link between elevated Lp(a) levels and a poor prognosis after PCI. A previous meta-analysis of observational studies reported an increased incidence of ISR in patients with elevated Lp(a) levels.20 Of the nine observational studies included, all patient cohorts were treated with a bare metal stent (BMS) or first-generation DES. A single-centre retrospective observational study looking at 2,292 patients with previous ISR-PCI identified a significantly higher risk of MACE and repeat revascularisation in patients with Lp(a) concentrations >30 mg/dL. The total cholesterol levels in this study were slightly higher in the elevated Lp(a) group (4.1 mmol/L vs. 3.8 mmol/L) but the prevalence of diabetes and high BMI was higher in the low Lp(a) group. The rate of BMS implantation was low (<3%) and similar amongst both groups. The use of intracoronary imaging was also low amongst both groups,19 which is one of the main limitations of the study, as the mechanism for stent failure could not be accurately assessed. One study from Japan specifically looked at new-generation DESs and the risk of recurrent MACE following PCI in patients with elevated Lp(a) concentrations. During three years of follow-up, the incidence of MACE was high, which was mainly driven by spontaneous MIs and unplanned revascularisations.21 A list of studies investigating the association of Lp(a) and outcomes after PCI are contained in table 1.
Table 1. Lp(a) as a predictor of MACE following PCI
| Study | Study population | Primary end point | Definition of elevated Lp(a) level used in study | Results |
| Zhang et al, 202319 | Patients with PCI for ISR | MACE and repeat revascularisation | >30 mg/dL | Increased risk of MACE and repeat revascularisation in high Lp(a) group |
| Yoon et al, 202115 | Patients with previous PCI | CV death, MI and ischaemic CVA, recurrent ischaemic events | >30 mg/dL | Increased ischaemic events in elevated Lp(a) level group |
| Qin et al, 201320 | Meta-analysis, patients with previous PCI to ISR and de novo lesions | Rate of ISR | N/A; meta-analysis of 9 studies | Increased ISR in patients with elevated Lp(a) levels |
| Liu et al, 202018 | Patients with low LDL levels following PCI | CV death, CVA, MI and repeat revascularisation | >118.0 mmol/L | Increased incidence of repeat revascularisation in high Lp(a) level group |
| Park et al, 201522 | Stable angina patients post-PCI | MI and revascularisation | ≥50 mg/dL | Increased rate of restenosis |
| Kimura et al, 202221 | Patient with PCI for de novo lesions | CV death, MI, stent thrombosis, unplanned revascularisation | ≥30 mg/dL | Higher incidence of MACE in Lp(a) levels >30 mg/dl |
| Key: CV = cardiovascular; CVA = cerebrovascular accident; CVD = cardiovascular disease; ISR = in-stent restenosis; LDL = low-density lipoprotein; Lp(a) = lipoprotein(a); MACE = major adverse cardiovascular events; MI = myocardial infarction; PCI = percutaneous coronary intervention | ||||
Conclusion
Lp(a) is considered a major risk factor for atherosclerotic CVD. Multiple studies have established an association between elevated Lp(a) levels and incident CAD in the general population. Moreover, observational data suggests that Lp(a) is associated with increased adverse events in patients with established CAD and may contribute to an increased rate of ISR in patients with previous PCI who are on optimal medical therapy. Perhaps the most convincing data on the mechanism of Lp(a) and worse CV outcomes comes from imaging studies, which show high-risk features of plaque vulnerability in patients with elevated Lp(a) concentrations.
Key messages
- Lipoprotein(a) (Lp[a]) is an independent risk factor for CV events in patients with established coronary artery disease (CAD)
- Elevated Lp(a) levels are associated with adverse plaque features in patients with established CAD
- Elevated Lp(a) levels are associated with increased risk of in-stent restenosis in patients with previous percutaneous coronary intervention
- Elevated Lp(a) levels are associated with degenerative aortic stenosis
- To date, there are no randomised controlled trials confirming a causality between elevated Lp(a) levels and recurrent CV events.
Conflicts of interest
Authors have received honoraria for their work on this supplement.
Pyotr Telyuk
Consultant Cardiologist
David Austin
Consultant Cardiologist
Paul Williams
Consultant Cardiologist
Academic Cardiovascular Unit, The James Cook University Hospital, Middlesbrough, TS4 3BW
Ahai Luvai
Consultant Cardiologist
Department of Clinical Biochemistry, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, NE1 6NDN
Azfar G Zaman
Clinical Professor of Cardiology
Department of Cardiology, The Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, Newcastle upon Tyne, NE1 6NDH
([email protected])
Articles in this supplement
Introduction
Lipoprotein(a) in atherosclerotic heart disease and familial hypercholesterolaemia
Lipoprotein(a) measurement – how, why and in whom?
Raised lipoprotein(a): real-world examples of communication and clinical management
References
1. Telyuk P, Austin D, Luvai A, Zaman A. Lipoprotein(a): insights for the practicing clinician. J Clin Med 2022;11:3673. https://doi.org/10.3390/jcm11133673
2. Rallidis LS, Pavlakis G, Foscolou A et al. High levels of lipoprotein (a) and premature acute coronary syndrome. Atherosclerosis 2018;269:29–34. https://doi.org/10.1016/j.atherosclerosis.2017.12.011 [Epub online ahead of print]
3. Reyes-Soffer G, Ginsberg HN, Berglund L et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2022;42:e48–e60. https://doi.org/10.1161/ATV.0000000000000147 [Epub online ahead of print]
4. Ugovšek S, Šebeštjen M. Lipoprotein(a) – the crossroads of atherosclerosis, atherothrombosis and inflammation. Biomolecules 2021;12:26. https://doi.org/10.3390/biom12010026
5. Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK. Lipoprotein(a): an independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J 2019;71:99–112. https://doi.org/10.1016/j.ihj.2019.03.004 [Epub online ahead of print]
6. Rehberger Likozar A, Zavrtanik M, Šebeštjen M. Lipoprotein(a) in atherosclerosis: from pathophysiology to clinical relevance and treatment options. Ann Med 2020;52:162–77. https://doi.org/10.1080/07853890.2020.1775287 [Epub online ahead of print]
7. Niccoli G, Chin D, Scalone G et al. Data on the lipoprotein (a), coronary atherosclerotic burden and vulnerable plaque phenotype in angiographic obstructive coronary artery disease. Data Brief 2016;7:1409–12. https://doi.org/10.1016/j.dib.2016.04.017
8. Muramatsu Y, Minami Y, Kato A et al. Lipoprotein (a) level is associated with plaque vulnerability in patients with coronary artery disease: an optical coherence tomography study. Int J Cardiol Heart Vasc 2019;24:100382. https://doi.org/10.1016/j.ijcha.2019.100382
9. Huded CP, Shah NP, Puri R et al. Association of serum lipoprotein(a) levels and coronary atheroma volume by intravascular ultrasound. J Am Heart Assoc 2020;9:e018023. https://doi.org/10.1161/JAHA.120.018023 [Epub online]
10. Cegla J, Neely RDG, France M et al. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. https://doi.org/10.1016/j.atherosclerosis.2019.10.011 [Epub online ahead of print]
11. Kaiser Y, Daghem M, Tzolos E et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol 2022;79:223–33. https://doi.org/10.1016/j.jacc.2021.10.044
12. Bowman L, Mafham M, Wallendszus K et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–39. https://doi.org/10.1056/NEJMoa1804988 [Epub online ahead of print]
13. Chasman DI, Shiffman D, Zee RY et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis 2009;203:371–6. https://doi.org/10.1016/j.atherosclerosis.2008.07.019 [Epub online ahead of print]
14. de Boer LM, Oorthuys AOJ, Wiegman A et al. Statin therapy and lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol 2022;29:779–92. https://doi.org/10.1093/eurjpc/zwab17
15. Yoon YH, Ahn JM, Kang DY et al. Association of lipoprotein(a) with recurrent ischemic events following percutaneous coronary intervention. JACC Cardiovasc Interv 2021;14:2059–68. https://doi.org/10.1016/j.jcin.2021.07.042
16. Schwartz GG, Ballantyne CM, Barter PJ et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-OUTCOMES randomized clinical trial. JAMA Cardiol 2018;3:164–8. https://doi.org/10.1001/jamacardio.2017.3833
17. O’Donoghue ML, Morrow DA, Tsimikas S et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol 2014;63:520–7. https://doi.org/10.1016/j.jacc.2013.09.042 [Epub online ahead of print]
18. Liu Y, Zeng Z, Yu X et al. Impact of lipoprotein(a) on long-term outcomes after percutaneous coronary intervention in patients with reduced low-density lipoprotein cholesterol. Rev Cardiovasc Med 2020;21:147–53. https://doi.org/10.31083/j.rcm.2020.01.5101
19. Zhang H, Zhang Y, Tian T et al. Association between lipoprotein(a) and long-term outcomes after percutaneous coronary intervention for lesions with in-stent restenosis. J Clin Lipidol 2023;17:458–65. https://doi.org/10.1016/j.jacl.2023.05.094 [Epub online ahead of print]
20. Qin S-y, Liu J, Jiang H-x, Hu B-l, Zhou Y, Olkkonen VM. Association between baseline lipoprotein (a) levels and restenosis after coronary stenting: Meta-analysis of 9 cohort studies. Atherosclerosis 2013;227:360–6. https://doi.org/10.1016/j.atherosclerosis.2013.01.014 [Epub online ahead of print]
21. Kimura T, Akahori H, Tanaka T et al. Impact of lipoprotein (a) on long-term outcome after percutaneous coronary intervention in the era of new generation drug-eluting stents. J Cardiol 2022;80:179–83. https://doi.org/10.1016/j.jjcc.2022.03.004 [Epub online ahead of print]
22. Park S-H, Rha S-W, Choi B-G et al. Impact of high lipoprotein(a) levels on in-stent restenosis and long-term clinical outcomes of angina pectoris patients undergoing percutaneous coronary intervention with drug-eluting stents in Asian population. Clin Exp Pharmacol Physiol 2015;42:588–95. https://doi.org/10.1111/1440-1681.12396
