More than 200 years have passed since William Withering’s ground-breaking work on the medical use of foxglove for the treatment of dropsy. Its derivative, digoxin, remains one of the most heavily debated drugs in cardiovascular medicine. While its use predated the era of randomised control trial evidence or even a complete understanding of its mechanism of action, it has endured against a changing scientific and therapeutic landscape. In this historical review, we explore its journey from ancient wisdom to becoming a cornerstone of heart failure (HF) therapy, including discovery of its pharmacological properties and an appraisal of early clinical studies. The development of newer therapies for HF during the late twentieth century, alongside concerns regarding digoxin’s toxicity, impact on mortality, and comparative efficacy, have led to a decrease in its use. Nevertheless, digoxin remains a useful treatment option for selected patients with atrial fibrillation and HF.
From folklore to botany to medicine to art
Botany may be considered the predecessor of modern pharmacopoeia: in antiquity, a number of plants served as therapeutic remedies.1 The earliest use of foxglove can be traced back to the Greek and Roman eras, based on reports that the ‘father of pharmacognosy,’ Pedanius Dioscorides, was aware of its effects.2 In Welsh and Irish folklore, foxglove was considered to protect against the evil eye and witchcraft.3 The first detailed account of the medicinal use of foxglove took place in the sixteenth century when Leonhard Fuchs (1501–1566) suggested that it may have diuretic properties when taken orally, and may serve as a remedy for wounds when used topically.3 Fuchs also coined the name ‘digitalis purpurea’, from the Latin word ‘digitus’, which is the Latin reference to the appearance of a plant that the Germans called ‘fingernut’ (meaning thimble). Two centuries later in 1710, William Salmon claimed it had the ability to cure tuberculosis.3 Around the same time, the potential for toxicity was also identified. Salmon wrote that ‘it produces weakness, induces vomiting and purges.’ In 1748, François Salerne published the results of experiments showing that turkeys overfed with foxglove developed convulsions.4 Consequently, its use remained relatively limited until the work of the British physician William Withering.

| Reproduced from W. Bond, after Carl Frederik von Breda, Public domain, via Wikimedia Commons |
William Withering was born in Shropshire, England, in March 1741, into a well-educated family surrounded by apothecaries and physicians (figure 1).5.6 He studied medicine at the University of Edinburgh and, following his marriage, moved to Birmingham where he practiced for the rest of his life. He was held in high esteem by numerous academics and physicians of that era, such as Jenner, Heberden, Hunter, Lavoisier, Parry and Linnaeus. His devotion to his work was such that even after contracting tuberculosis in 1783, he continued to practice medicine until his death in 1799.5,6 At the time of his death, he was described in one account as ‘the flower of English physicians.’7
William Withering had a keen interest in botany and it was during his trips to the countryside where he first encountered the therapeutic properties of foxglove. As he mentions:
In the year 1775 my opinion was asked concerning a family receipt for the cure of the dropsy. I was told that it had long been kept a secret by an old woman in Shropshire, who had sometimes made cures after the more regular practitioners had failed. I was informed also, that the effects produced were violent vomiting and purging; for the diuretic effects seemed to have been overlooked. This medicine was composed of twenty or more different herbs; but it was not very difficult for one conversant in these subjects to perceive, that the active herb could be no other than the Foxglove.8
Over the next decade, he continued to investigate the effects of the plant and in 1785, he published An Account of the Foxglove, describing his experience of its use in 163 patients.8 This was one of the first scientific treatise on the treatment of disease written in the English language. In this account, Withering not only described the utility of foxglove for treating dropsy – a clinical syndrome resembling heart failure (HF) consistent of anasarca, pleural effusions and breathlessness – but also its mode of extraction, precise measurements for making an infusion and expected side effects. Even though atrial fibrillation (AF) was not formally described until 1935, Withering may have also alluded to patients with AF and HF as being those most likely to benefit:
If the pulse be feeble or intermitting, the countenance pale, the lips livid, the skin cold, the swollen belly soft and fluctuating, or the anasarcous limbs readily pitting under the pressure of the finger, we may expect the diuretic effect to follow in a kindly manner.8

| La Nuit étoilée. Vincent van Gogh. 1889. Museum of Modern Art (New York City). Creative Commons Attribution-Share Alike 4.0 |
Withering’s publication was received with great acclaim; however, the use of digitalis remained sporadic throughout much of the nineteenth century due to differing opinions on its utility and safety. Other well-known physicians, including Fothergill and Balfour, avoided digitalis due to concerns about its toxicity at high doses. Many physicians also experimented with digitalis for other conditions it could not improve (fever, epilepsy, goitre, scrofula), reinforcing the scepticism regarding its utility.4 Conversely in some circles in France, it was frequently used and may be referred to as ‘the opium of the heart,’ due to its ability to decrease the heart rate.3 It is speculated that digitalis toxicity was responsible for the vivid yellow tones observed in Vincent van Gogh’s later paintings, such as the renowned Starry Night (figure 2).9

| Portrait of Dr Gachet. Vincent van Gogh. 1890. https://commons.wikimedia.org/wiki/File:Portrait_of_Dr._Gachet.jpg |
Xanthopsia, an overriding yellow bias in vision, is one of features of digitalis toxicity originally described by William Withering (table 1).9 Van Gogh is thought to have suffered from epilepsy and may have been treated with digitalis by his French physician Paul Gachet. Interestingly, the only remaining portrait of Gachet by van Gogh displays him holding a sprig of foxglove (figure 3).10 It is worth remembering that before Withering’s account, dropsy had no specific treatment and was nearly always fatal. Hence, despite mixed opinions among medical professionals, the public perception was generally positive. In his later published book Botany, Withering shares a poem written by Miss Sarah Hoare (1767–1855) in 1818, whose father had been treated with digitalis for congestive HF:3
The foxglove’s leaves, with caution given,
Another proof of favouring Heav’n
Will happily display;
The rapid pulse it can abate;
The hectic flush can moderate
And, blest by Him whose will is fate,
May give a lengthen’d day.
Table 1. Digoxin side effects
| Common | Arrhythmias (ventricular ectopy, non-sustained VT, SVT) Cardiac conduction disorders (bradycardia due to sinus node exit and/or AV block) Cerebral impairment Diarrhoea Dizziness Eosinophilia Nausea Skin reactions Vision disorders (e.g. xanthopsia, blurred vision) Vomiting |
| Uncommon | Depression |
| Rare or very rare | Decreased appetite Asthenia Confusion Gynaecomastia Headache Malaise Psychosis Thrombocytopaenia |
| British National Formulary – last update: 20/02/2024 Key: AV = atrioventricular; SVT = supraventricular tachycardia; VT = ventricular tachycardia |
|
Although William Withering’s contributions to medicine extended beyond digitalis (he was also involved in the characterisation of scarlet fever as an infectious disease), his publication of digitalis’ medicinal effects remains his most distinguished legacy.5,6 It was also the cause of a bitter dispute with his contemporary Ernest Darwin, leading to the fallout of their relationship.5 Nevertheless, the pride he felt towards this discovery can be appreciated from his only known portrait where he is seen holding a foxglove (figure 1). His epitaph at the Edgbaston Old Church is also decorated with carvings of digitalis.5
Sir James Mackenzie and digitalis becomes digoxin
Sir James Mackenzie is considered to be one of the pioneers of cardiac electrophysiology and another pivotal figure in the history of digitalis.11 At the beginning of the twentieth century, following the development of the electrocardiograph, major advances had been made in the understanding of cardiac arrhythmias. Among his seminal findings, Mackenzie is credited with demonstrating the efficacy of digitalis for the treatment of delirium cordis (an irregular pulse) and was among the first to postulate a vagal effect of the drug.12 In collaboration with the pharmacologist and physiologist Arthur Robertson Cushny, Mackenzie asserted that ‘in cases where the rhythm of the heart was normal, digitalis had little effect upon the rate.’13 However, other investigators of the time held differing views, e.g. Henry Christian in Boston wrote ‘digitalis has a striking effect on those changes in the patients which are brought about by cardiac insufficiency, and this effect appears irrespective of whether or not the pulse is irregular.’14 Mackenzie’s views were also opposed by his colleague, Thomas Lewis, who served as Editor of the journal Heart, and rejected the publication of Mackenzie’s manuscript regarding digitalis and vagal tone in 1921, leading to a fallout between the two contemporaries.11
The transition from digitalis to digoxin occurred after 1930. Emerging evidence suggested that digitalis lanata leaves were far more potent than those of digitalis purpurea, leading to the extraction of a new substance (similar to gitoxin by Sydney Smith, a researcher from Burroughs Wellcome, UK), which was later named digoxin.15 Amidst continued debate surrounding the optimal form of administration (i.e. digitoxin, digoxin or digitalis leaves),16 in 1954, the US Food and Drug Administration granted a license to the first branded digoxin tablets (Lanoxin™) as a treatment for HF and AF.17
Discovering the mode of action
Alongside Withering’s original description of the diuretic effects of digitalis, his account also alluded to an inotropic effect, wherein digoxin exhibited ‘a power over the motion of the heart.’8 By 1900, following a series of physiological experiments in frogs, digitalis had been shown to increase the force of contraction of the isolated heart. Several leading figures argued that this enhanced cardiac contractility was digoxin’s primary effect, whilst others, including Cushny, Mackenzie and Lewis, advocated a primary mode of action on cardiac conducting tissues. Lewis wrote:
Those who regarded digitalis as a cardiac stimulant mistake its character: its chief action is to rest the heart. To the heart foxglove is not a tonic but powerfully hypnotic.18
Today it is appreciated that both modes of action are correct. Digoxin is a cardiac glycoside (a name given by Gerhardt in 1852),19 positive inotrope (increases the force of myocardial contraction), negative chronotrope (slows the heart rate) and antiarrhythmic (reduces conductivity at the atrioventricular [AV] node).19,20 It may also inhibit renin release.20
The central mechanism underlying digoxin’s action, namely inhibition of the sarcolemmal sodium/potassium adenosine triphosphatase (Na+/K+ ATPase) pump, was only discovered by Schatzmann in 1953.21 After digoxin binds to and blocks Na+/K+ ATPase, the resulting increase in intracellular Na+ leads to a secondary rise in intracellular Ca2+ concentration via increased activity of the Na+/Ca2+ exchanger, which removes intracellular Na+ in exchange for Ca2+ influx (from work by Wilbrandt and Koller in 1948, among others).4 In cardiac myocytes, this leads to increased force of contraction (described by Heilbrun and Wiercinski in 1945), yielding the positive inotropic action of digoxin.19 The negative chronotropic effect is presumed to be due to a combined activation of the vagus nerve (parasympathetic action) and inhibition of the sympathetic nervous system. AV node inhibition, in conjunction with the rise in intracellular Ca2+, leads to prolongation of phase IV and phase 0 of the cardiac action potential, increasing the AV node refractory period. Slower conduction through the AV node produces a slower ventricular rate.4,19 Inhibition of the renal Na+/K+ ATPase may also reduce Na+ reabsorption and renin secretion via tubuloglomerular feedback.19
From wuthering heights to Withering confidence
Allen B Weisse, a respected cardiologist and medical historian, once commented that at the beginning of the twentieth century, ‘if you knew how to use digitalis, in whatever form you preferred, you knew just about all you could about treating heart failure and atrial fibrillation.’16 However, the enthusiasm for digoxin waned over subsequent years and it has never been far from controversy.
After Withering’s practice of extracting digitalis using alcohol extract or tincture was superseded by pills made from powered digitalis leaf, there was initial variation in the potency of such pills. This often meant that the correct dose had to be deduced by trial and error in each patient and the process had to be repeated with each batch of pills. An important development was the introduction of biological standardisation procedures by Houghton in 1886, as well as availability of purified preparations of digoxin by the 1920s. The establishment of a digoxin radioimmunoassay in the 1960s made it possible to assess the bioavailability of digoxin. In 1971, however, an investigation of products marketed in several countries reported differences in digoxin bioavailability between brands. In 1972, it became known that batches of Lanoxin™ tablets had been released into the UK market which resulted in approximately twice the plasma levels of the appropriate dose and could have led to catastrophic effects.20 Despite determining a safe therapeutic range for digoxin in the mid-1970s, monitoring of plasma levels was still not routinely performed until later.22,23
It is notable that the use of digoxin had preceded any randomised evidence or even basic understanding of its mechanism of action. The development of clinical trial methodology, whereby relative values of different drug treatments or of the same drug in different clinical situations could be more objectively assessed, heralded renewed investigation of digoxin. Small, uncontrolled and withdrawal studies were performed between 1970 and 1990 with favourable results. However, in 1988, the first president of the British Society for Heart Failure, Philip Poole-Wilson, highlighted a growing concern that,
…whilst the therapeutic propensities of digoxin as a negative chronotrope and positive inotrope are not under question, the universal use of digoxin in heart failure even in the presence of newer, safer and efficacious medications with research basis should come under more extensive investigation.24,25
In particular, he stipulated the need for dedicated controlled comparative trials to examine the effects of digoxin on mortality and quality of life against newer agents, such as diuretics and angiotensin-converting enzyme (ACE) inhibitors.24,25
The current state of affairs
A detailed discussion of the evidence for digoxin’s safety and efficacy is outside the scope of this historical review. However, it is pertinent to briefly review factors contributing to lingering debate.
Most of the current controversy regarding digoxin relates to its use in HF in sinus rhythm. To date, the only large, randomized trial evaluating the efficacy of digoxin versus placebo on hard outcomes in patients with HF in sinus rhythm, was performed by the Digitalis Investigation Group (DIG), enrolling 6,800 patients and concluding in 1997.26 The DIG investigators reported a significant reduction in HF hospitalisations with digoxin and a neutral effect on mortality, both in the main trial and in the subset of patients deemed to have ‘diastolic HF’ (results summarised in table 2).26–28
Table 2. Focused digoxin-specific randomised controlled trials
| Study | Year | Condition | Comparators | Population | Results |
| DIG trial26 | 1997 | HF (HFrEF*) | Digoxin vs. placebo | Digoxin: 3,397 Placebo: 3,403 |
Primary outcome: No significant difference in all-cause mortality (digoxin: 34.8%, placebo: 35.1%; RR: 0.99; 95% CI: 0.91–1.07; p=0.80). Key secondary outcomes: Trend (not statistically significant) towards reduced death due to worsening HF (digoxin: 11.5%, placebo: 13.2%; RR: 0.88; 95% CI: 0.77–1.01; p=0.06). Reduction in HF hospitalisations in the digoxin arm (26.8%) vs. placebo (34.7%) (RR: 0.72; 95% CI: 0.66–0.79; p<0.001). |
| DIG – ancillary study27 | 2006 | HF (HFpEF**) | Digoxin vs. placebo | Digoxin: 492 Placebo: 496 |
Primary outcome: No difference in the combined outcome of HF hospitalisation or HF mortality (digoxin: 21%, placebo: 24%; HR: 0.82; 95% CI: 0.63–1.07; p=0.136). Key secondary outcomes: No difference in all-cause mortality (digoxin: 23%, placebo: 23%; HR: 0.99; 95% CI: 0.76–1.28; p=0.925) or HF mortality (digoxin: 6%, placebo: 7%; HR: 0.88; 95% CI: 0.54–1.43; p=0.598). No difference in CV hospitalisation (digoxin: 49%, placebo: 45%; HR: 1.10; 95% CI: 0.92–1.32; p=0.301). Trend (not statistically significant) towards reduced HF hospitalisation (digoxin: 18%, placebo: 22%; HR: 0.79; 95% CI: 0.59–1.04; p=0.094). Trend (not statistically significant) towards increased hospitalisation for unstable angina (digoxin: 17%, placebo: 13%; HR: 1.37; 95% CI: 0.99–1.91; p=0.061). |
| RATE-AF28 | 2020 | AF | Digoxin vs. bisoprolol | Digoxin: 80 Bisoprolol: 80 |
Primary outcome: No difference in patient-reported quality of life at 6 months (normalised SF-36 PCS) (AMD: 1.4; 95% CI: −1.1 to 3.8;p=0.28). Key secondary outcomes: No difference in SF-36 domains or AFEQT score at 6 months. Reduction in median NT-proBNP concentration at 6 months with digoxin vs. bisoprolol but no significant between-group difference. Adverse events (1+) were less common with digoxin (25%) vs. bisoprolol (64%) (p<0.001). No significant difference in resting or exertional heart rate between arms. |
| * HFrEF: LVEF ≤40% ** HFpEF: LVEF ≥50% Key: AF = atrial fibrillation; AFEQT = Atrial Fibrillation Effect on Quality of Life; AMD = adjusted mean difference; CI = confidence interval; CV = cardiovascular; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; NT-proBNP = N-terminal of the prohormone of B-type natriuretic peptide; RATE-AF = Rate Control Therapy Evaluation in Permanent Atrial Fibrillation Team; RR = risk ratio; SF-36 PCS = 36-item Short Form Health Survey Physical Component Survey |
|||||
Since then, several aspects of the DIG trial have been scrutinised. Firstly, a significant proportion of enrolled patients were already taking digoxin at trial entry; it has been speculated the result may have been different if the whole cohort was digoxin-naïve.29 Secondly, as the DIG trial was conducted in the early nineties before routine use of beta blockers, mineralocorticoid receptor antagonists, angiotensin receptor-neprilysin inhibitors, sodium-glucose co-transporter-2 inhibitors and device-based therapy, its relevance for contemporary HF management is questioned (although ACE inhibitor trials were also performed before the beta blocker era and are still advocated today). Finally, the dose of digoxin used was 250 µg and target serum concentration ≤2 ng/ml; it was later reported that concentrations >1 ng/ml were associated with adverse outcomes.30
After publication of the DIG trial, a number of post-hoc reports, systematic reviews and meta-analyses cast doubts on the clinical efficacy of digoxin with new concerns raised regarding increased mortality risk with long-term digoxin use in patients with HF31 and/or AF.32,33 Such reviews have contributed to the portrayal of digoxin in the national media as an ‘old and dirty’ drug.34 It is important to note, however, that these studies were based on observational data (administrative databases or registries) or secondary (retrospective) analyses of trials, which have inherent risks of confounding.35 For example, digoxin is often prescribed for older and sicker patients who have higher pre-treatment risk of mortality.36 Whilst statistical analyses can minimise bias in the treatment effect estimate, they do not eliminate it.37 Even judicious use of methods such as propensity score matching cannot replace randomisation. Hence, interpretation of these non-randomised studies should be considered hypothesis-generating.
In recent years, for the treatment of HF, digoxin has been superseded by several newer therapies that boast greater efficacy culminating in a paradigm shift in HF treatment from inotropic support towards neurohumoral modulation. This can be seen by the decreasing trend in digoxin use among participants in HF clinical trials (figure 438–44),36 and observational studies.45,46 It is estimated that 20% of patients with HF are currently prescribed digoxin, a large proportion of whom have concomitant AF.47–49 In the absence of contemporary randomised data, its use has been de-emphasised in HF guidelines where it is currently considered a second- or third-line agent for patients with HF and reduced ejection fraction (left ventricular ejection fraction ≤40%) in sinus rhythm who have persistent symptoms, despite combining other treatments (class IIb).50
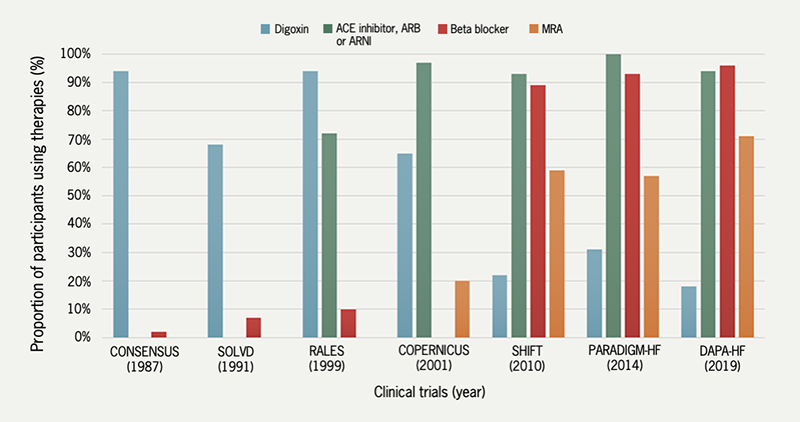
| Key: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; CONSENSUS = Cooperative North Scandinavian Enalapril Survival Study; COPERNICUS = Carvedilol Prospective Randomized Cumulative Survival; DAPA-HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; MRA = mineralocorticoid receptor antagonist; PARADIGM-HF = Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure; RALES = Randomized Aldactone Evaluation Study; SHIFT = Systolic Heart Failure Treatment with the If Inhibitor Ivabradine; SOLVD = Studies of Left Ventricular Dysfunction |
The most common indication for digoxin use today is for the control of rapid ventricular rates in patients with AF, with and without HF (class IIa).51 Given its unique combination of a positive inotropic and negative chronotropic effect, it is frequently used where blood pressure is low and rate control is sub-optimal, especially in patients with AF and HF with reduced ejection fraction. However, even for this indication, the lack of a digoxin effect on heart rate during physical exercise and the absence of a prognostic relationship between heart rate and outcomes in patients with AF and HF are considered a disadvantage. In the European Society of Cardiology HF guidelines, digoxin is only recommended for use in patients with concomitant AF when other therapeutic options (namely beta blockers) are insufficient or poorly tolerated (class IIa).50 Both HF and AF guidelines (table 3)50–55 also highlight the narrow therapeutic window. Target serum concentrations of digoxin should be maintained at ≤1.2 ng/ml to avoid harm and physicians are advised to remain vigilant for signs of toxicity, especially in elderly patients or those with chronic kidney disease (as digoxin is predominantly excreted via the kidneys).50
Table 3. Summary of NICE, ESC and AHA guidelines relevant to AF, HF and digoxin
| Organisation | Year | Condition | Recommended indications | Dose |
| NICE52 | 2018 | HF | As 2nd line in worsening or severe HFrEF*, despite first-line treatment No routine measurement of levels is needed |
Nil specified |
| NICE53 | 2021 | AF | As monotherapy in non-paroxysmal AF if no or little physical activity or other options cannot be used As combination therapy in continuing symptoms Do not offer digoxin for prevention of post-operative AF |
Nil specified |
| ESC50 | 2021 | HF | As 2nd line therapy in addition to a beta blocker if AF is present and resting HR remains >110 bpm May also be considered in HFrEF* if the patient is in SR |
Oral daily dose: 62.5–250 µg |
| Target serum concentration: <1.2 ng/ml | ||||
| ESC51 | 2020 | AF | As 2nd line therapy in addition to a beta blocker | IV dose: 500 µg bolus (750–1500 µg over 24 hours in divided doses) |
| Oral daily dose: 62.5–250 µg | ||||
| AHA54 | 2022 | HF | As 2nd line therapy for worsening HF symptoms despite guideline-directed medical treatment (i.e. beta blocker, ACE inhibitor/ARB/ARNI, SGLT2 inhibitor, MRA) | Oral daily dose: 125–250 µg |
| Target serum concentration: 0.5–0.9 ng/ml | ||||
| AHA55 | 2023 | AF | As 2nd line therapy where a beta blocker or non-dihydropyridine calcium-channel blocker have been ineffective or are contraindicated | IV dose: 250–500 µg over several minutes (with repeat doses of 250 µg 6 hourly – maximum: 1500 µg over 24 hours) |
| Oral daily dose: 62.5–250 µg | ||||
| Target serum concentration: <1.2 ng/ml | ||||
| *HFrEF: defined as heart failure with a reduced left ventricular ejection fraction ≤40% Key: ACE = angiotensin-converting enzyme; AF = atrial fibrillation; AHA = American Heart Association; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; bpm = beats per minute; ESC = European Society of Cardiology; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HR = heart rate; IV = intravenously; MRA = mineralocorticoid receptor antagonist; NICE = National Institute for Health and Care Excellence; SGLT2 = sodium-glucose co-transporter-2; SR = sinus rhythm |
||||
Looking forwards, to the best of our knowledge, there are no registered randomised trials of digoxin and the prospect of one for this low-cost generic drug seems unlikely. However, a clinical trial of the alternative, digitoxin, is currently recruiting. The DIGIT-HF trial (Digitoxin to Improve Outcomes in Patients with Advanced Chronic Heart Failure) is a multi-centre, randomised, double-blind, placebo-controlled trial, aiming to enrol 2,190 patients to evaluate whether digitoxin can reduce the risk for all-cause mortality and/or hospital admission for worsening HF (NCT03783429).56
Digoxin – quo vadis?
In 2009, an editorial posed the question ‘Quo vadis? (where are you going)’ and 15 years later, the role of digoxin remains contentious.57 Nevertheless, despite criticism, fierce debate and time, this centuries-old drug continues to be used by HF and arrhythmia specialists worldwide, albeit with declining rates. Digoxin may even find itself a new clinical indication; in preclinical research, cardiac glycosides, including digoxin, are being investigated for potential anticancer effects.58,59 Hence, while the foxglove and its derivatives may not hold the dominant position they once held, it has been suggested that their role has been ‘modified but not relegated to the garden or the medical history book.’35 At least for now, William Withering’s great discovery and legacy stand firm.
After all, in spite of opinion, prejudice or error, Time will fix the real value upon this discovery, and determine whether I have imposed upon myself and others, or contributed to the benefit of science and mankind.8
Key messages
- Whilst medicinal use of digitalis dates back to antiquity, William Withering is credited with the first systematic investigation of its use for the treatment of dropsy in 1785, leading to widespread recognition
- Digoxin is derived from the foxglove plant Digitalis lanata. Its previous form, digitalis, was derived from Digitalis purpurea
- Digoxin is a cardiac glycoside with positive inotropic and negative chronotropic mechanisms of action; it may also inhibit renin release
- Whist its use has been debated in recent years, digoxin remains a treatment option for selected patients with atrial fibrillation and heart failure.
Articles in this supplement
Digoxin: a look back and a look forward
The modern-day role of digoxin in heart failure and atrial fibrillation – benefits and limitations
Digoxin: monitoring, limitations of its use, and managing toxicity
Conflicts of interest
XK and RZ have received an honorarium for working on this supplement.
Funding
RZ is supported by a National Institute for Healthcare Research (NIHR) Advanced Fellowship award (NIHR302961)
Xenophon Kassianides
Specialty Registrar in General Practice and Honorary Lecturer
Hull University Teaching Hospitals NHS Trust and Hull York Medical School, Hull
Rosita Zakeri
Senior Clinical Lecturer and Honorary Consultant Cardiologist
King’s College London and King’s College Hospital and Guy’s and St Thomas’ NHS Foundation Trusts, London
References
1. Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism 2008;57(suppl 1):S3–9. https://doi.org/10.1016/j.metabol.2008.03.001
2. Lewis LD. Early human studies of investigational agents: dose or microdose? Br J Clin Pharmacol 2009;67:277–9. https://doi.org/10.1111/j.1365-2125.2009.03397.x
3. Jacobs MS. The history of digitalis therapy. Ann Med Hist 1936;8:492–9.
4. Wray S, Eisner DA, Allen DG. Two hundred years of the foxglove. Med Hist 1985;(suppl 5):132–50. https://doi.org/10.1017/s0025727300070551
5. Lee MR. William Withering (1741–1799): a Birmingham lunatic. Proc R Coll Physicians Edinb 2001;31:77–83.
6. Silverman ME. William Withering and an account of the foxglove. Clin Cardiol 1989;12:415–8. https://doi.org/10.1002/clc.4960120714
7. Roddis LH. William Withering and the introduction of digitalis into medical practice (part II). Ann Med Hist 1936;8:185–201.
8. Withering W. An Account of the Foxglove, and Some of its Medical Uses: With Practical Remarks on Dropsy and Other Diseases. Cambridge: Cambridge University Press; 2014, first published: 1785.
9. Lee TC. Van Gogh’s Vision: Digitalis intoxication? JAMA 1981;245:727–9.
10. Somberg JC. Van Gogh and digitalis. Am J Cardiol 2020;136:164–5. https://doi.org/10.1016/j.amjcard.2020.09.009 [Epub online ahead of print]
11. Silverman ME. From rebellious palpitations to the discovery of auricular fibrillation: contributions of Mackenzie, Lewis and Einthoven. Am J Cardiol 1994;73:384–9. https://doi.org/10.1016/0002-9149(94)90013-2
12. Moss AJ. Introductory note on Sir James Mackenzie. Ann Noninvasive Electrocardiol 2005;10:387. https://doi.org/10.1111/j.1542-474X.2005.20050711a.x
13. Mackenzie J. The action of digitalis on the human heart. Proc R Soc Med 1908;1(Ther Pharmacol Sect):29–32.
14. Christian HA. The use of digitalis other than in the treatment of cardiac decompensation. JAMA 1933;100:789–92. https://doi.org/10.1001/jama.1933.02740110001001
15. Smith S. Digoxin, a new digitalis glucoside. J Chem Soc 1930:508–10.
16. Weisse AB. A fond farewell to the foxglove? The decline in the use of digitalis. J Card Fail 2010;16:45–8. https://doi.org/10.1016/j.cardfail.2009.08.001 [Epub online ahead of print]
17. Gona SR, Rosenberg J, Fyffe-Freil RC, Kozakiewicz JM, Money ME. Review: failure of current digoxin monitoring for toxicity: new monitoring recommendations to maintain therapeutic levels for efficacy. Front Cardiovasc Med 2023;10:1179892. https://doi.org/10.3389/fcvm.2023.1179892
18. Lewis T. On cardinal principles in cardiological practice: a British Medical Association lecture delivered to the Sheffield Division, October 24th, 1919. Br Med J 1919;2:621–5. https://doi.org/10.1136/bmj.2.3072.621
19. Cuthbert JJ, Clark AL. Rate limiting agents (digoxin). In: Clark AL, Gardner RS, Theresa A. McDonagh TA (eds.). Oxford Textbook of Heart Failure (2nd edn). Oxford: Oxford University Press, 2022;651.
20. Wade OL. Digoxin 1785–1985. Two hundred years of digitalis. J Clin Hosp Pharm 1986;11:3–9. https://doi.org/10.1111/j.1365-2710.1986.tb00822.x
21. Schatzmann HJ. Cardiac glycosides as inhibitors of active potassium and sodium transport by erythrocyte membrane. Helv Physiol Pharmacol Acta 1953;11:346–54.
22. Carruthers SG, Kelly JG, McDevitt DG. Plasma digoxin concentrations in patients on admission to hospital. Br Heart J 1974;36:707–12. https://doi.org/10.1136/hrt.36.7.707
23. Selzer A. Role of serum digoxin assay in patient management. J Am Coll Cardiol 1985;5(suppl A):106A–10A. https://doi.org/10.1016/s0735-1097(85)80469-1
24. Poole-Wilson PA. Digitalis: dead or alive? Cardiology 1988;75(suppl 1):103–9. https://doi.org/10.1159/000174449
25. Poole-Wilson PA, Robinson K. Digoxin – a redundant drug in congestive cardiac failure. Cardiovasc Drugs Ther 1989;2:733–41. https://doi.org/10.1007/BF00133201
26. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–33. https://doi.org/10.1056/NEJM199702203360801
27. Ahmed A, Rich MW, Fleg JL et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary Digitalis Investigation Group trial. Circulation 2006;114:397–403. https://doi.org/10.1161/CIRCULATIONAHA.106.628347 [Epub online ahead of print]
28. Kotecha D, Bunting KV, Gill SK et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–508. https://doi.org/10.1001/jama.2020.23138
29. Opie LH. Digitalis, yesterday and today, but not forever. Circ Cardiovasc Qual Outcomes 2013;6:511–3. https://doi.org/10.1161/CIRCOUTCOMES.113.000544
30. Ahmed A, Rich MW, Love TE et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006;27:178–86. https://doi.org/10.1093/eurheartj/ehi687
31. Chang KY, Giorgio K, Schmitz K et al. Effect of chronic digoxin use on mortality and heart failure hospitalization in pulmonary arterial hypertension. J Am Heart Assoc 2023;12:e027559. https://doi.org/10.1161/JAHA.122.027559
32. Turakhia MP, Santangeli P, Winkelmayer WC et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol 2014;64:660–8. https://doi.org/10.1016/j.jacc.2014.03.060
33. Freeman JV, Reynolds K, Fang M et al. Digoxin and risk of death in adults with atrial fibrillation: the ATRIA-CVRN study. Circ Arrhythm Electrophysiology 2015;8:49–58. https://doi.org/10.1161/CIRCEP.114.002292
34. Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 2015;36:1831–8. https://doi.org/10.1093/eurheartj/ehv143 [Epub online ahead of print]
35. Whayne TF Jr. Clinical use of digitalis: a state-of-the-art review. Am J Cardiovasc Drugs 2018;18:427–40. https://doi.org/10.1007/s40256-018-0292-1
36. Cuthbert JJ, Clark AL. Learning module. Digoxin: current clinical uses and management of toxicity. Br J Cardiol [online] 2023. Available at: https://bjcardio.co.uk/2023/06/digoxin-current-clinical-uses-and-management-of-toxicity/ (accessed 7 Mar 2024)
37. Aguirre Davila L, Weber K, Bavendiek U et al. Digoxin-mortality: randomized vs. observational comparison in the DIG trial. Eur Heart J 2019;40:3336–41. https://doi.org/10.1093/eurheartj/ehz395
38. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–35. https://doi.org/10.1056/NEJM198706043162301
39. Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN; SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–91. https://doi.org/10.1056/NEJM199209033271003. Erratum in: N Engl J Med 1992;327:1768.
40. Pitt B, Zannad F, Remme WJ et al.; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17. https://doi.org/10.1056/NEJM19990902341100
41. Packer M, Coats AJ, Fowler MB et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. https://doi.org/10.1056/NEJM200105313442201
42. Swedberg K, Komajda M, Böhm M et al.; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. https://doi.org/10.1016/S0140-6736(10)61198-1. Erratum in: Lancet 2010;376:1988.
43. McMurray JJV, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. https://doi.org/10.1056/NEJMoa1409077
44. McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
45. Alahmed AA, Lauffenburger JC, Vaduganathan M et al. Contemporary trends in the use of and expenditures on digoxin in the United States. Am J Cardiovasc Drugs 2022;22:567–75. https://doi.org/10.1007/s40256-022-00540-x. [Epub online ahead of print]
46. Angraal S, Nuti SV, Masoudi FA et al. Digoxin use and associated adverse events among older adults. Am J Med 2019;132:1191–8. https://doi.org/10.1016/j.amjmed.2019.04.022. [Epub online ahead of print]
47. Veenis JF, Brunner-La Rocca H-P, Linssen GCM et al. Atrial fibrillation in chronic heart failure patients with reduced ejection fraction: the CHECK-HF registry. Int J Cardiol 2020;308:60–6. https://doi.org/10.1016/j.ijcard.2020.03.001. [Epub online ahead of print]
48. Crespo-Leiro MG, Anker SD, Maggioni AP et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–25. https://doi.org/10.1002/ejhf.566
49. NICOR. National Heart Failure Audit (NHFA). Available at: https://www.nicor.org.uk/heart-failure-heart-failure-audit/ (accessed 7 Mar 2024)
50. McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
51. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612. Erratum in: Eur Heart J 2021;42:507. Erratum in: Eur Heart J 2021;42:546–7. Erratum in: Eur Heart J 2021;42:4194.
52. National Institute for Healthcare and Excellence (NICE). Guidance: chronic heart failure in adults: diagnosis and management [NG106]. Available at: https://www.nice.org.uk/guidance/ng106 (accessed 11 Mar 2024)
53. National Institute for Healthcare and Excellence (NICE). Guidance: atrial fibrillation: diagnosis and management [NG196]. 2021. Available at: https://www.nice.org.uk/guidance/ng196 (accessed 11 Mar 2024)
54. Heidenreich PA, Bozkurt B, Aguilar D et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2022;145:e876–94. https://doi.org/10.1161/CIR.0000000000001062 [Epub online ahead of print]
55. Joglar JA, Chung MK, Armbruster AL et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1–156. https://doi.org/10.1161/CIR.0000000000001193 [Epub online ahead of print]
56. Bavendiek U, Berliner D, Dávila LA et al. Rationale and design of the DIGIT-HF trial (digitoxin to improve outcomes in patients with advanced chronic heart failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail 2019;21:676–84. https://doi.org/10.1002/ejhf.1452 [Epub online ahead of print]
57. Cleland JGF, Cullington D. Digoxin: quo vadis? Circ Heart Fail 2009;2:81–5. https://doi.org/10.1161/CIRCHEARTFAILURE.109.859322
58. Elbaz HA, Stueckle TA, Tse W, Rojanasakul Y, Dinu CZ. Digitoxin and its analogs as novel cancer therapeutics. Exp Hematol Oncol 2012;1:4. https://doi.org/10.1186/2162-3619-1-4
59. Calderón-Montaño JM, Burgos-Morón E, Orta ML, Maldonado-Navas D, Garcia-Dominguez I, López-Lázaro M. Evaluating the cancer therapeutic potential of cardiac glycosides. Biomed Res Int 2014;2014:794930. https://doi.org/10.1155/2014/794930
