A Bayer 2023 Cardiovascular Exchange Summit post-meeting report
The following expert faculty members have received an honorarium for participating at the meeting
| Faculty member | Affiliation |
| Professor Amitava Banerjee | Professor of Clinical Data Science, Institute of Health Informatics, University College London; Consultant Cardiologist, University College London Hospitals and Barts Health NHS Trust |
| Professor Sir Mark Caulfield | Professor of Clinical Pharmacology, Queen Mary University of London & Vice-Principal for Health for Queen Mary’s Faculty of Medicine and Dentistry |
| Professor Keith Fox | Professor of Cardiology, University of Edinburgh & British Heart Foundation Professor of Cardiology (Emeritus) |
| Dr Matt Kearney | Executive Director for Cardiovascular Health, UCL Partners |
| Professor Kamlesh Khunti | Professor of Primary Care Diabetes and Vascular Medicine, University of Leicester |
| Professor Vijay Kunadian | Professor of Interventional Cardiology, Newcastle University and Honorary Consultant Interventional Cardiologist, Freeman Hospital Newcastle upon Tyne Hospitals NHS Foundation Trust |
| Dr Guy Lloyd | Consultant Cardiologist, Barts Heart Centre and Honorary Secretary; British Cardiovascular Society |
| Dr Kieran McCafferty | Consultant Nephrologist, Barts Health NHS Trust; Honorary Senior Lecturer, Queen Mary University of London; Barts Health NIHR CRF Clinical Director; UKKRC CKD and Diabetes Co-Chair |
| Professor Nicholas S Peters | Professor of Cardiology & Head of Cardiac Electrophysiology, Imperial College, London |
| Mr Piers Ricketts | Chief Executive of Eastern AHSN |
| Professor Ganesh Subramanian | Clinical Associate Professor, University of Nottingham; Consultant in Stroke Medicine, Nottingham University Hospitals NHS Trust |
| Dr Clare Taylor | Academic General Practitioner, Nuffield Department of Primary Care Health Sciences, University of Oxford |
| Professor Raj Thakkar | GP, Buckinghamshire, CKD Representative & President of the PCCS |
| Key: AHSN = Academic Health Science Networks (now known as Health Innovation Network; CKD = chronic kidney disease; CRF = Cardiovascular Research Foundation; GP = general practitioner; NHS = National Health Service; NIHR = National Institute for Health and Care Research; PCCS = Primary Care Cardiovascular Society; UCL = University College London; UKKRC = United Kingdom Kidney Research Consortium | |
The following external authors were involved in the generation and approval of this report
|
LCW Consulting Bayer acknowledges that the workshop feedback outlined in the report has been contributed to by delegates in attendance at the meeting who were not paid an honorarium. The Bayer medical team have medicolegally reviewed the document for compliance with the Association of the British Pharmaceutical Industry Code of Practice and for technical accuracy. |
The Bayer Cardiovascular Exchange Summit 2023 was organised by Bayer in collaboration with the British Cardiovascular Society (BCS) and the Primary Care Cardiovascular Society (PCCS), providing a forum for the exchange of knowledge and experience from generalists and specialists across cardiovascular disease (CVD) management. The meeting focused on how innovation and leadership could be used to drive change within the National Health Service (NHS) for the benefit of patients, population health and the wider NHS. Key areas where innovation could transform CVD management and NHS service provision were explored and are summarised in this report:
- A holistic approach to CVD prevention and management, and patient empowerment
- Delivering co-ordinated and patient-centred care: innovative models of working
- Innovations in licensing and life science regulation
- Embedding innovation within clinical practice
Innovations in healthcare are being developed at pace and both healthcare professionals (HCPs) and payers need to adapt and embrace new medicines, technologies and services, embedding them into clinical pathways. Barriers that delay the availability of these tools need to be addressed and full use should be made of the enablers that exist to ensure innovations are available to all who could benefit from them. CVD pathways need to be transformed at a system level, addressing inequalities and regional disparities and with greater integration between primary and secondary care. Patients should be at the heart of all new healthcare innovations and should be considered at every step of developmental and regulatory pathways.
The outputs from the Bayer Cardiovascular Exchange Summit 2023 provide valuable insights into how innovation can be used to transform the management of CVD, representing perspectives from stakeholders including HCPs working across primary and secondary care, as well as patient representatives.
Introduction
In an era of rapid technological advancement and evolving healthcare needs, the National Health Service (NHS) needs to embrace innovation, integrating new technologies, evidence-based practices, and novel approaches into clinical workflows to ensure consistent high-quality care and optimised patient outcomes. Building on the success of the 2022 Cardiovascular Exchange Summit,1 the 2023 Bayer Cardiovascular Exchange Summit provided a forum for the exchange of ideas and experiences from primary care generalists and secondary care specialists with an interest in cardiovascular (CV) medicine, as well as patient representatives. They explored how innovation and leadership could be used to drive change within the NHS for the benefit of patients. The two-day meeting was organised in partnership with the British Cardiovascular Society (BCS) and the Primary Care Cardiovascular Society (PCCS), with an expert faculty spanning cardiovascular, renal and stroke multidisciplinary teams.
The meeting addressed key areas where innovation could play a vital role in transforming cardiovascular disease (CVD) management and NHS service provision:
- A holistic approach to CVD prevention and management, and patient empowerment
- Delivering co-ordinated and patient-centred care: Innovative models of working
- Innovations in licensing and life science regulation
- Embedding innovation within clinical practice
Members of the speaking faculty shared their experience and examples of best practice during plenary sessions, providing background context and setting the scene for further, in-depth conversations during workshops and panel discussions in which delegates shared their own ideas and experiences, discussing how innovation could be used to transform pathways and services within the NHS. The multidisciplinary nature of the audience allowed voices to be heard from across primary and secondary care and different disciplines, including patient perspectives, providing a valuable insight into how innovations are being and could be adopted across the spectrum of CV care services within the NHS.
Both the BCS and PCCS are committed to working in partnership with patients, the pharmaceutical industry and relevant organisations with aligned strategic aims to identify the current challenges in CVD management within the NHS and to identify solutions to address these.
This report summarises the key messages from the meeting.
A holistic approach to CVD prevention and management, and patient empowerment
Growing evidence suggests that cardiovascular (CV), renal and metabolic (CVRM) conditions, such as heart failure, chronic kidney disease (CKD), inflammation, thrombosis and diabetes, are intertwined (figure 1). Therefore, there is a need for a holistic approach to patient care.2–9 Both the mechanisms of these conditions and their risk factors need to be understood to identify high-risk patients and provide optimal care.
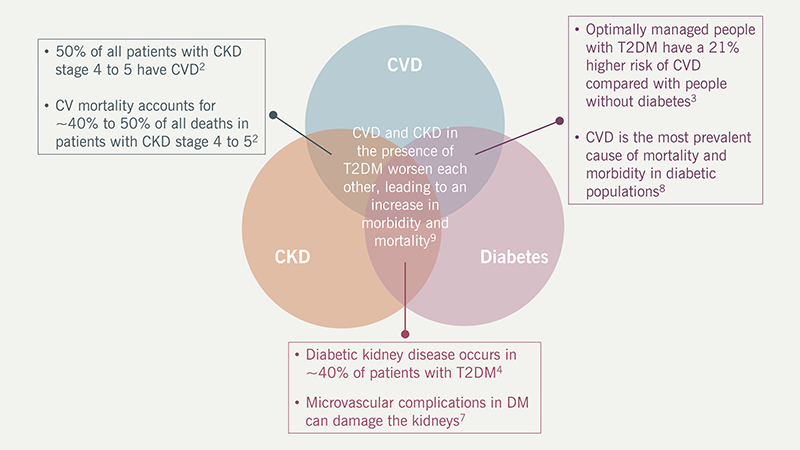
| Key: CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; CVRM = cardiovascular, renal and metabolic; T2DM = type 2 diabetes mellitus |
There needs to be a shift towards a preventative health system
The risk factors for CVD are well established and understood (figure 2). Many of these are modifiable, presenting the opportunity for people to reduce their CV risk through early and proactive lifestyle and pharmacological interventions.10,11 However, many patients with CVD or risk factors for CVD remain undiagnosed or sub-optimally treated.12 The 2019 NHS Long Term Plan states that CVD ‘is the single biggest area where the NHS can save lives over the next 10 years.’ Identifying undetected hypertension and hypercholesterolaemia, and consequent optimisation of blood pressure and cholesterol levels is outlined within the Long Term Plan as a key strategy to achieve the ambition of preventing 150,000 heart attacks, strokes and dementia cases over the 10-year period.13
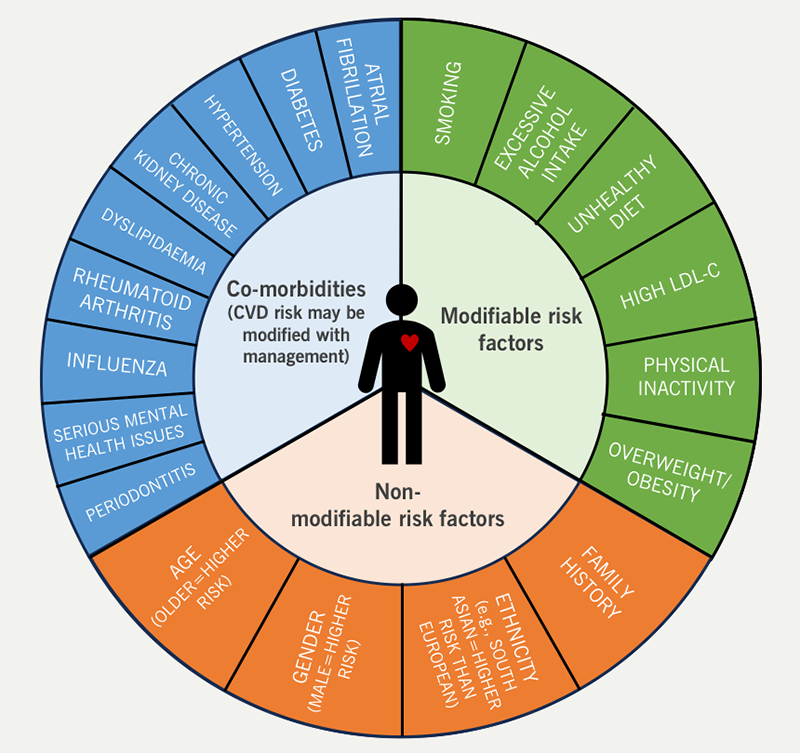
| Key: CVD = cardiovascular disease; LDL-C = low-density lipoprotein cholesterol |
Hypertension is the number one risk factor globally for mortality and affects 15 million people in the UK, of which around 4.8 million remain undiagnosed.12,14 Blood pressure control is often achievable, and technological advances such as home blood pressure monitoring can empower patients to take control of blood pressure management.15 However in England, around 30% of patients with hypertension are not optimally treated.16 If even 80% of people with hypertension were optimally managed, around 14,000 heart attacks and strokes and over 4,400 deaths would be prevented in just three years, with savings to the NHS of around £150 million. Similarly, a large number of CV events and deaths could be saved with associated cost savings to the NHS, if cholesterol levels were optimised in patients with established CVD.17 This is recognised in the 2023/24 Quality and Outcomes Framework (QOF), which includes two indicators to ensure that all patients with established CVD or CKD receive optimal treatment to reduce levels of low-density lipoprotein cholesterol (LDL-C).18
Patients should feel engaged and take ownership of their condition(s)
Early identification of people with risk factors for CVD and understanding their risk profiles can enable healthcare professionals (HCPs) to co-develop an individualised care plan with them. HCPs can support patients to understand the value and purpose of minimising their risk and the potential benefits and harms of the treatments offered to them. Patient education is key to engagement and adherence. Providing patients with the information and tools to change their behaviour and lifestyle, while tailoring treatment according to the individual patient’s needs and preferences in line with the National Institute for Health and Care Excellence (NICE) guidance,19 will help them take ownership of their condition and adhere to the treatments agreed with their HCPs. However, this requires time and the NHS is currently struggling with staff shortages.20
Secondary prevention is crucial for the prevention of further CVD events. Aggressive treatment should be offered to lower blood pressure and LDL-C levels, along with antithrombotic and antidiabetic therapies for those who would benefit from them.21 Both new and established therapies that have been shown to reduce CV death, all-cause mortality and strokes which are approved by NICE need to be used more effectively across the UK to provide individualised care. There needs to be a particular focus on pro-actively identifying and managing patients with frailty (including dementia) or those in the last year of life, given that over-medicalisation can cause harm.
Inequalities and disparities need to be understood, addressed and resourced to make CVD healthcare more equitable
Late diagnosis, suboptimal treatment and unwarranted variation in the management of high-risk conditions for CVD is widespread and entrenched within the NHS.22 CVD is a major driver of health inequalities, accounting for around a fifth of the life-expectancy gap between the most- and least-deprived areas of England.23 It is critical to acknowledge that usually silent high-risk CVDs are hard to manage in real-world general practice, with complexity, multimorbidity and time pressures being the norm. Demand in primary care is overwhelming, with little spare capacity; contracts and incentives are not aligned to evidence. Therefore, pathways need to be transformed at a system level and made simpler, with inequalities and regional disparities addressed.
Example in practice – University College London (UCL) Partners Proactive Care Frameworks
UCL Partners, a Health Innovation Network (HIN), have developed a series of proactive care frameworks to support primary care teams to manage patients with long-term CV and respiratory conditions. These provide systemic, structured support to enable primary care practices to prioritise clinical activity by stratifying patients at highest risk, deploying the wider workforce to reduce the workload for GPs and improve personalised care for patients. The frameworks cover five CV conditions (atrial fibrillation [AF], hypertension, hypercholesterolaemia, type 2 diabetes and heart failure), as well as asthma and chronic obstructive pulmonary disease (COPD). Framework resources include slide decks, practical guides and digital resources for clinicians to support management, addressing day-to-day challenges in care, as well as guidance, protocols and training support for Additional Role Reimbursement Scheme (ARRS) roles to deliver support for education, self-management and behavioural change. Acknowledging the difference in care pathways across the country, the frameworks can be implemented in a flexible way to fit the variety of clinical settings seen in England.24
The UCL Partners Proactive Care Framework for Hypertension has been used in Newham, a socially deprived area of London with a population comprising 73.2% non-white ethnic minority groups, to address the health inequality gap in the area. The risk stratification and prioritisation tools enabled patients to be seen by appropriate professionals in line with their CV risk: patients identified as being at high risk of CVD were seen by clinical pharmacists, advanced nurse practitioners and GPs, whereas patients identified as being at moderate risk of CVD were seen by nursing colleagues and healthcare assistants (HCAs). Patients at low risk of CVD were supported by well-being coaches and HCAs. This stratification helped to free-up GP time through making use of the wider team staff, as well as empowering patients, particularly those in the lower CV risk categories, to take control of their own health and well-being. The frameworks provided guidance around HCP roles and patient goals, resulting in proactive and targeted treatment for patients and reducing the disease burden in the population.25
Digital technologies are increasingly being used for diagnosis and management of CVD
Culture and innovations for the diagnosis and monitoring of CVD and its risk factors have an important role in the future of CVD prevention. Advances in digital technologies, including wearable devices, provide opportunities to identify and treat asymptomatic patients at an early stage and can demonstrate value and cost-effectiveness, reducing the dependency on HCPs and also the need for more costly interventions as the condition progresses.26 Artificial intelligence (AI)-powered tools are also proving to be increasingly valuable, building in treatment components and linking to pathways.27,28 However, the processes to purchase and adopt technologies in the NHS needs urgent review. Furthermore, the medical workforce needs to be enabled to embrace these innovations and be upskilled on the use of digital technology, while ensuring patients who lack digital literacy are not disadvantaged.
Delivering co-ordinated and patient-centred care: innovative models of working
HCPs need to work in partnership with patients, viewing them as a whole person rather than just a person with a single disease
Many patients with CVD have several other long-term conditions (multiple long-term conditions or multimorbidity) and, therefore, do not follow simple algorithmic pathways. Policy changes are needed to facilitate integrated care between primary and secondary care settings, with a focus on patient-centred, personalised care, considering frailty, multi-morbidity and polypharmacy.
Personalised care provides people with choice and control over the way their care is planned and delivered, empowering them to make decisions. Care should be based on what matters to the patient and their individual strengths and needs.29 Self-management, supported by education can help empower patients with CV, metabolic and renal diseases to take ownership of their conditions. Furthermore, patient advocacy and support groups, patient steering groups and digital technologies, such as healthcare apps, can help to involve patients in their own care, empowering them to make changes to their lifestyle and improve adherence to treatments (figure 3).30–32
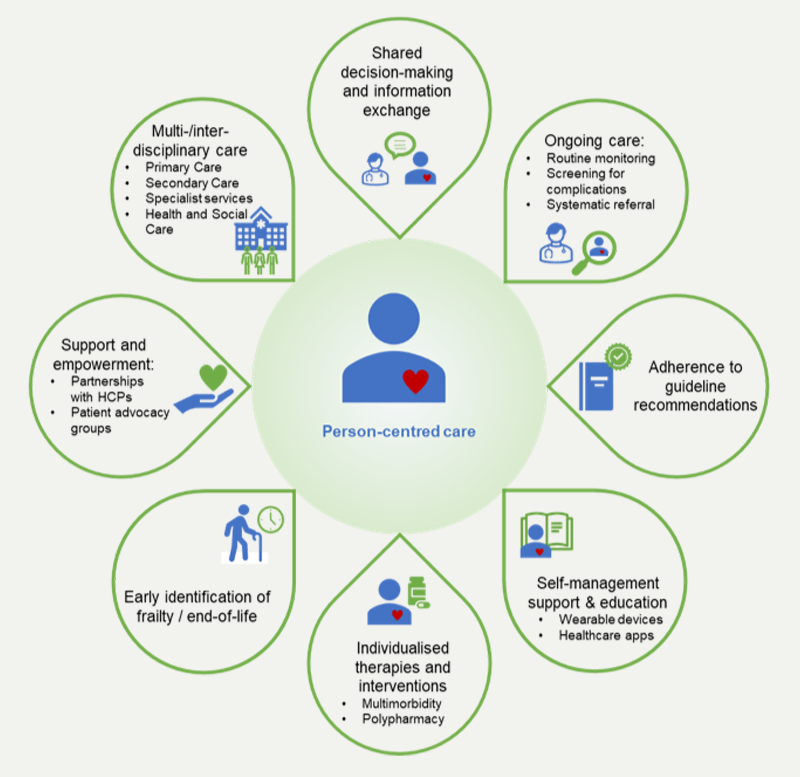
| Key: CVD = cardiovascular disease; HCPs = healthcare professionals |
Clinical pathways for CVD diagnosis and management need to be transformed at a system level and made simpler
Around-the-clock integrated working between primary and secondary care HCPs, across disciplines, and between patients and HCPs is essential for the provision of personalised care for patients, especially for those with complex comorbidities, to ensure continuity of care.36 Integrated working can streamline and transform CVD patient pathways, improving both patient-centred care and patient outcomes.22
Example in practice: hub-and-spoke model for acute stroke care
A hub-and-spoke thrombectomy model in which patients with suspected stroke are admitted to the nearest hyperacute stroke centre (spoke) and patients with confirmed stroke are transferred to the thrombectomy centre (hub), has been trialled within the NHS and shown to be an effective way of providing patients with rapid access to imaging and thrombectomy. The use of automated analysis software across hub-and-spoke centres is also being trialled to determine if this may reduce delays in decision making.37 Furthermore, the use of telestroke networks can enable stroke consultants based in hub centres to provide spoke centres with support and expertise and teleconsultations, and also remotely assess computed tomography (CT) images to enable time-sensitive decisions around patient treatment to be made.38 The stroke pathway can also be streamlined through the use of machine-learning algorithms to predict which patients are suitable for thrombectomy,39 pre-hospital video triage to assess stroke patients on the scene, as well as the use of AI to support image interpretation.40 Together, these innovations can speed up the time-sensitive decision-making process enabling patients to receive imaging and treatments, such as thrombectomy, more quickly, potentially saving lives or reducing the severity of disability following a stroke. The implementation of hub-and-spoke pathways and the use of imaging software incorporating AI and decision-making support tools to streamline the stroke pathway and rapidly identify patients who would benefit from thrombectomy are recommended in the 2022 Getting It Right First Time (GIRFT) Stroke National Report as part of a series of recommendations for improvements in stroke service delivery and patient care.41
HCPs need to identify where change is needed within clinical pathways and embrace new ways of working to provide sustainable high-quality personalised care for all patients. They should break away from working in silos and instead work in partnerships, taking full advantage of innovations and enablers that can help transform pathways. Health Innovation Networks (HINs, formerly Academic Health Science Networks, AHSNs) work with healthcare organisations and businesses to support the adoption and spread of innovation within the NHS to improve services for patients, enable NHS efficiencies and support economic growth. Over 2.3 million patients have benefitted from HIN programmes to date, including those with CVD.
Example in practice: Detect, Protect, Perfect Programme for AF
AF-related strokes are more likely to be fatal or severely debilitating than non-AF-related strokes.42 Anticoagulation treatment can reduce the risk of stroke in people with AF by up to 60%;43 however, 9% of people in England with AF and high stroke risk are not receiving anticoagulation.44 Supported by funding from NHS England, the national Detect, Protect, Perfect programme was delivered by all AHSNs between April 2018 and March 2020. The programme aimed to raise awareness of AF and pulse rhythm testing to identify undiagnosed AF (‘Detect’), support HCPs to offer optimal anticoagulation to all patients who would benefit (‘Protect), and support patients with their anticoagulation medication as well as supporting clinicians to review patients with AF (‘Perfect’).45
A whole systems approach was adopted, with HINs providing quality improvement tools and resources, as well as supporting the sharing of best practice across the country. Over the course of the programme, an estimated 12,000 AF-related strokes have been avoided with approximately 2,900 lives and £158 million in NHS costs and £105 million in social care costs saved.45
There are also established HIN programmes for the optimisation of blood pressure and lipid management, and familial hypercholesterolaemia,46 as well as a recently launched programme for the management of heart failure.47
Therapeutic inertia exists in CVD management and needs to be addressed
Once patients have been identified and are being treated for CVD, it is important to ensure they are followed up regularly and remain optimally managed. Therapeutic inertia is usually described as the failure to advance therapy when appropriate to do so, but also includes the failure to de-intensify therapy when appropriate, for example in frailty or last year of life, when the harms associated with therapies may outweigh the benefits. HCPs, patients and the healthcare system can all contribute to therapeutic inertia (figure 4).48,49
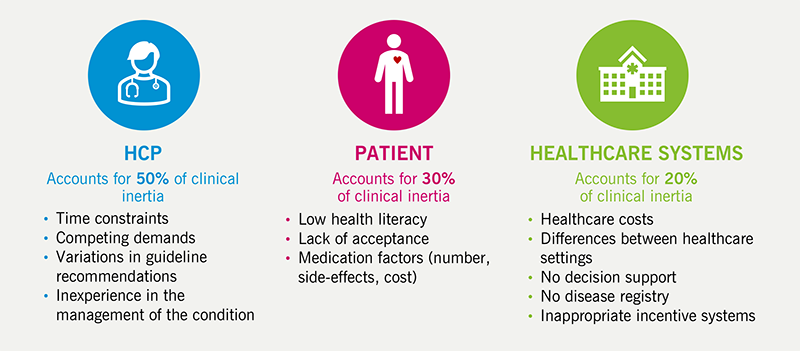
| Key: HCP = healthcare professionals |
Measures including education for HCPs, adherence to guidelines (but tailored to the individual e.g. those with frailty), development of quality measures, effective use of information systems, contractual enablers, demand management, adoption of value-based medicine and motivation and support for patients around self-management and adherence may all help combat therapeutic inertia.51,52 Furthermore, empowering pharmacists and nurses to initiate and intensify treatment may also be an effective approach to mitigate therapeutic inertia.53
Innovations in clinical research, licensing and life science regulations
The process by which innovations and clinical trial findings are implemented into clinical practice and embedded into patient care pathways needs to be accelerated
Clinical trials provide the data and evidence needed to demonstrate that new innovations are beneficial for patients. However, the cohorts of those trialled and the applicability to real-world settings need to be considered; there can be long delays in embedding these innovations into clinical practice and making them available for use within the NHS. The NHS Long Term Plan set out ambitions to speed up the pipeline for developing innovations and the adoption of innovation into ‘business-as-usual’ within the NHS, to enable proven and affordable new techniques and technologies reach patients faster and to reduce variation.13 There are a number of schemes in place to fast-track the licensing of new medicines and technologies, including the Innovative Licensing and Access Pathway (ILAP),54 the Early Access to Medicines Scheme (EAMS)55 and the Accelerated Access Collaborative (AAC).56 These aim to provide patients with faster access to innovations, based on a balance of risks and benefits and for conditions where there is a clear and urgent unmet need. In addition, there are opportunities for international regulatory collaborations. The Access Consortium aims to provide accelerated access to high-quality medicines by maximising international cooperation and reducing duplication through work-sharing initiatives.57 Conversely, the International Recognition Procedure (IRP) will allow medicines approved in Australia, Canada, Europe, Japan, Switzerland, Singapore and the USA that meet certain criteria to undergo a fast-tracked Medicines and Healthcare Products Regulatory Agency (MHRA) review to obtain and/or update UK marketing authorisations.58
Research should be embedded within clinical care and can encourage collaboration and the integration of primary and secondary care
Access to clinical research should be facilitated for patients and routinely embedded in clinical care. Research can provide income to bolster increasingly strained NHS budgets with commercial sponsors often paying for nurses, doctors and drugs, benefitting more than just the patients taking part in the trial, and can also help in the recruitment and retention of staff and boost staff morale.59 There is also evidence that hospitals with a greater level of research funding have lower patient mortality rates.60 Patient experience of clinical research is generally positive, with those who participate potentially benefitting from access to treatments they may otherwise have not been eligible for. They also receive continuity of care with the same HCPs, frequent and high-quality contact with HCPs, and clear explanations. Furthermore, patients feel they are making a contribution and helping others.59,61 However, there is inequality in access to research for patients across the UK.62 This needs to be addressed to enable all patients to access appropriate clinical trials and drugs, and to ensure that patient samples are representative of the patient population. Every patient should be considered a potential research participant and efforts should be made to ensure patients want to take part in clinical research.
Clinical trials should give significant consideration to patient-reported outcome measures (PROMs)
Patients should be at the heart of all new healthcare innovations and should be considered at every step of the developmental and regulatory pathway, from trial design through to regulatory approval. While improving outcomes and mortality is a key focus for any new therapy, it is also important to consider the impact on the patient’s quality of life and their perception of the burden of disease. PROMs are often considered a less important outcome within trial design. However, they can provide important information for patients, families and clinicians, as well as trialists, regulators, commissioners and industry, about the patient’s perception of their own health, including functional status and health-related quality of life. From the patient perspective, the lived experience is extremely important and PROMs are the only way to capture this within a trial.63 PROMs are increasingly being included as end points in clinical trials,64–67 and are also now being used in health economic modelling and regulatory decision making.68,69
The MHRA have published guidance around the use of real-world evidence (RWE) in clinical studies and randomised controlled trials to support regulatory submissions.70,71 Use of RWE, including electronic health records (EHRs), PROMs and disease and patient registries to improve recruitment and aid regulatory decision making could accelerate the development approval processes for new therapies and technologies so they can be made available to patients more quickly. Use of EHRs can provide a large study population from which multiple datasets can be obtained across many different variables and have been used, for example, to identify patterns in CVD risk in selected populations and the impact of the coronavirus disease 2019 (COVID-19) pandemic on CVD services.72–75 Furthermore, combining EHR data with machine learning identified disease subtypes in both CVD and CKD may inform clinical risk prediction and future trial design.76,77
Embedding innovation within clinical practice
The embedding of findings from clinical trials into clinical practice and guidelines needs to be accelerated
In order for patients to benefit from new medicines, technologies and services, innovations need to be embraced by both clinicians and payers and embedded into clinical pathways where they are made accessible to all patients who might benefit from them and improve outcomes in the real world. The implementation of new technologies into clinical practice can be challenging, with multiple barriers that delay the availability of these tools for HCPs and patients within the NHS.
Small pilot schemes can be used to demonstrate the effectiveness of innovations; however, these do not always translate to wider implementation. An alternative to pilot schemes is direct implementation of technologies into clinical care.
Example in practice – AI-enabled stethoscope for the detection of HFrEF
Detection of heart failure in the primary care setting is a priority in the NHS Long Term Plan.13 However, over 80% of patients with heart failure are identified upon emergency admission to hospital, incurring an overall extra longitudinal cost of £2,485 per patient.28 Use of an AI algorithm with an electrocardiogram (ECG)-enabled stethoscope* produced an adequate ECG reading in over 93% of cases and showed an 85% sensitivity for detecting a left ventricular ejection fraction (LVEF) <40% in 1,050 patients attending for transthoracic echocardiogram. This point-of-care diagnostic tool has the potential to transform the heart failure pathway, enabling low-cost screening for heart failure with reduced ejection fraction (HFrEF) in a community setting and addressing the predominant diagnosis of heart failure through emergency admission.27 The clinical benefit and health economic impact of the ECG-enabled stethoscope and its usability and acceptability in clinical practice is now being measured in a large-scale cluster randomised study embedded in direct care across 200 GP practices in North West London and North Wales; 50% of practices are using the ECG-enabled stethoscope and the other 50% are using routine care. Where the device detects signs of cardiac disease, GPs will be able to rapidly perform further investigations and initiate treatment, if appropriate.78,79 Through this study, the technology, along with its potential benefits, is being made available to patients whilst its effectiveness in the general population is being assessed.
* Not currently approved for use in the UK.
Example in practice: CVDACTION
UCL Partners have developed CVDACTION, a smart data tool designed to support primary care to deliver a step change in preventative care. Six key metrics for CVD conditions – atrial fibrillation, blood pressure, cholesterol, CKD, non-diabetic hyperglycaemia and diabetes – are brought together, integrating the results of around 85 searches into user-friendly, actionable dashboards in order to display gaps, opportunities and inequalities in preventive care. This provides GP practices with patient-identifiable information so that clinicians can act on the data to prioritise and optimise care where needed.80
Key take-home messages
- There should be greater systematic focus on CVD prevention with patients identified and diagnosed in a timely fashion to reduce CV risk down the line
- Primary care is on the front line and crucial for prevention; by the time the patient reaches secondary care, the prevention window has been eroded
- Primary care cannot do it all: CVD prevention needs to be owned across integrated care boards and wider society, e.g. citizens, employers, schools, etc., and be empowered by technology
- Patients need to be engaged and empowered to take ownership of their CVD condition(s) with self-management encouraged and supported
- Digital healthcare technologies are being developed at pace and HCPs need to be empowered to access them and adapt their working practices, recognising the benefits for both patients and the health service
- A holistic approach is needed for CVRM management with personalised, patient-centric care, particularly focusing on early diagnosis and optimisation at one end, and the avoidance of ‘protocol hypnosis’ in patients who have severe frailty, to ensure optimal outcomes for all patients. There is also a need to identify a care coordinator who may be a GP, a specialist or even a carer
- Pathways need to be transformed with greater integration between primary and secondary care and between disciplines, incorporating new technologies and innovative approaches to care that have been shown to be beneficial and cost effective
- Research should be embedded within clinical care and HCPs should strive to take part in research projects and also encourage patients who may benefit from trials to participate in them
- Good data is key to transforming health care pathways and driving better outcomes. It needs to be systematically captured and made available to both primary and secondary care to facilitate integrated care
- Implementation of new medicines and technologies within clinical care needs to be accelerated with full use being made of the enablers available and adopted at a national level to ensure innovations are available to all who could benefit from them
- Inequalities in healthcare still exist and must be addressed and care must be taken to ensure these are not increased through the increased use of digital technologies
- Patients should be at the heart of all we do with their individual needs and preferences considered throughout every stage of health care, from trial design, through regulatory approval and in implementation of innovations into clinical practice.
Appendix
A holistic approach to cardiovascular disease (CVD) prevention and management, and patient empowerment
Example in practice – University College London (UCL) Partners Proactive Care Frameworks
A holistic approach to CVD prevention and management is needed that is integrated across both primary and secondary care and throughout the integrated care system (ICS)
- Integrated CVD care across the multi-disciplinary team (MDT) and across different geographical areas should make use of metabolic/cardio-renal clinics and virtual clinics, where appropriate, to deliver holistic patient-focused management
- Complex factors, such as frailty, multimorbidity and polypharmacy, are additional, essential considerations when managing CVD – personalised care is required and health and social care also needs to be considered
- CVD management should be placed into the context of how people live their lives with multimorbidity and frailty considered and incorporated into routine care
- An individual within the MDT should take primary responsibility for coordinating care and be held accountable when managing patients with CVD
- Both the patient and HCPs need to understand and know who this person is
- This should be someone that the patient feels they can trust, who is accessible and who can both communicate and listen to the patient, providing two-way information.
Clinical pathways for CVD diagnosis and management need to be transformed at a system level and made simpler
- Current commissioning arrangements (with most funding held in primary care) are not always appropriate for MDT approaches to care1
- Better use should be made of nurses, pharmacists and community healthcare services
- The point of diagnosis is key to accessing management – this is where there is a delay in the system and access issues can lead to inequalities
- Barriers to diagnosis need to be addressed (e.g. deployment of point-of-care testing and triage of appropriate referrals to specialists).
Inequalities and disparities between genders, ethnic groups and across different regions of the UK need to be understood, addressed and resourced to make CVD healthcare more equitable
- Quality data can be used to change systems and improve outcomes – this needs to be representative, capturing the real-world population
- Understanding local and national data is key to identifying areas where improvement is needed, what the problems are, and how they can be addressed.
There needs to be a shift towards a preventative health system
- Integrated care boards (ICBs) must invest more in early risk factor and disease detection and optimisation
- Controlling multifactorial risk factors is key to preventing CVD
- The public need to be engaged and educated from a young age around CVD risk and how it can be reduced
- There needs to be a partnership between HCPs and the public around CVD prevention.
Digital technologies are increasingly being used for the diagnosis and management of CVD
- More work is required to upskill the medical workforce on the use of digital technology, and the medical community needs to adapt and embrace these innovations
- Wearable technologies present a fantastic opportunity for prevention and behaviour modification that could reduce the risk of CVD and the need for diagnosis and management, placing power into the hands of patients and providing benefits for both patients and the health service
- Digital technologies require digital literacy which may exclude some patient groups from accessing these resources
- AI-powered tools will be increasingly valuable, building in treatment components and linking to pathways
- Guidelines are important for the adoption of technologies; however, they are slow to develop – mechanisms are needed to adopt technologies into clinical practice more quickly
- Guidelines often require real-world evidence, which creates a catch-22 situation as approval for use is needed for implementation into clinical practice and there are challenges for each ICB to invest in technology – more processes are needed for national adoption
- Guidelines should not be considered the finished product, but rather the start of best practice, and should empower HCPs to do better than we are currently doing
- Value and cost-effectiveness of technologies need to be demonstrated to both the population and the health service
- HCPs need to feel empowered and permitted to use technologies
- Proactive communication with ICBs can enact enablers and create locally commissioned services, making best use of available resources.
Patients should feel engaged and involved in the management of their condition(s) so that they can take ownership and become a driver of their own care
- Patient education is key to engagement and adherence; patients should be given the information and tools to change their behaviour and lifestyle, with a focus on secondary prevention strategies and the importance of medication adherence
- Patients need to know what their targets are and understand the risks associated with missing these targets
- Supporting self-management can reduce pressure on the health service and free up HCPs’ time for other patients
- Treatment should be tailored to the patient with their individual needs and preferences considered, in line with NICE guidance.19
Clinical inertia exists in CVD management and needs to be addressed
- Patients need to be identified more efficiently and treatment intensified or de-intensified as and when is appropriate, to maintain optimal management of their conditions.
Delivering co-ordinated and patient-centred care: innovative models of working
Innovations in licensing and life science regulation
The process by which innovations and clinical trial findings are implemented into clinical practice and embedded into patient care pathways needs to be accelerated
- HCPs need to be enabled and permitted to use newly licensed medications, so it is important that they are not held back by ICB bureaucracy leading to red tape and variation
- Getting treatments to patients more quickly will be beneficial for communities and can save money in the long-term
- Access to drugs/medical devices needs to be equitable.
Consideration should be given to ways in which patient recruitment can be facilitated
- Every patient should be considered a potential research participant and efforts should be made to ensure patients who wish to take part in clinical research are enabled to do so
- Clinicians should embed research opportunities within standard care to facilitate patient access to clinical trials, referring when appropriate to other sites/colleagues to enlist patients into trials and leveraging primary care (e.g. screening for studies)
- Clear inclusion/exclusion pathways are needed and pre-screening and understanding the patient group is very important to ensure appropriate patients are recruited
- The sample of patients should be representative of the patient population, making use of translation tools where needed to recruit patients who don’t speak English.
Key success factors for patient retention in clinical trials
- Patients need to be enabled to feel part of the research group and feel involved – relationships are important
- HCPs need to be kind, flexible and approachable with good communication and take time to explain things to the patient.
Research should be embedded within clinical care and can encourage collaboration and the integration of primary and secondary care
- Most interventions research is delivered in secondary care but many CVD/CKD patients are solely managed in primary care and could miss out on the benefits of taking part in research
- Significant research also occurs in primary care but the capacity of primary care physicians to increase research opportunities for patients, given their competing demands, to deliver care is a challenge
- Pharmacists, nurses and other HCPs should be encouraged and empowered to become principal investigators (PIs) in trials (with a consultant as co-PI to provide support)
- Models of remuneration for research in primary care need to be addressed.
Trial design impacts the speed of the approval process
- Clinical trials should be designed to support the embedding of innovations into clinical practice
- Clinical leadership on trials is crucial
- There should be continuous learning and education throughout the clinical trial process.
Clinical trials should give significant consideration to patient-reported outcome measures (PROMs) which provide a patient’s perspective on their own health
- Patients should be involved in clinical trial design from the start of the process to ensure the outcomes that matter to them are considered and assessed
- Patients want treatments to provide improvement in their quality of life as well as a reduction in morbidity and mortality
- PROMs are increasingly being included as secondary or tertiary end points in clinical trials, however, they do not usually play a key role in regulatory evaluations.
Embedding innovation within clinical practice
We need to accept that we are living in a new digital world and get ahead of this, making better use of and adapting to technology
- NHS leaders should engage with the people developing technologies to ensure they are fit for purpose and help with genuine clinical unmet needs
- HCPs need to be informed as to what technologies are validated and available for use.
Robust leadership is needed at all levels along with a clear case for change to bring in investment and the resource to deliver that change
- Evidence needs to be presented to ICBs to make the case for change at the ICB/network level
- There is a need to upskill colleagues and mobilise community champions to drive change and improve efficiency.
There is a need to accelerate and embed the findings from clinical trials into clinical practice and update clinical guidance
Acknowledgements
Bayer would like to thank the chairs, faculty and delegates for their opinions, enthusiasm and engagement throughout the meeting.
Professor Kamlesh Khunti is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC).
Funding
The creation of this report was funded by Bayer plc.
Conflicts of interest
All faculty members received an honorarium for participating at the meeting.
Professor Kamlesh Khunti has acted as a consultant, speaker or received grants for investigator-initiated studies for Astra Zeneca, Bayer, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Pfizer, Roche and Applied Therapeutics.
Professor Vijay Kunadian has received honoraria from Bayer, CSL Behring and Medtronic.
Dr Kieran McCafferty has acted as a consultant or speaker for or received grants from AstraZeneca, Bayer, Fresenius, Oncacare, Napp and Vifor.
Professor Raj Thakkar has acted as a consultant or speaker for AstraZeneca, Abbott, Amgen, Bayer, Boehringer Ingelheim, Daiichi Sankyo and Novartis.
References
1. Fuat A, Gale C, Lloyd G et al. Inspiring change within the NHS to improve collaborative working and patient care across cardiovascular disease. Br J Cardiol 2023;30(suppl 3):S3–12. https://doi.org/10.5837/bjc.2023.s10
2. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–72. https://doi.org/10.1161/CIRCULATIONAHA.120.050686
3. Wright AK, Suarez-Ortegon MF, Read SH et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings. Circulation 2020;142:1925–36. https://doi.org/10.1161/CIRCULATIONAHA.120.046783
4. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–45. https://doi.org/10.2215/CJN.11491116
5. Christiansen CF, Schmidt M, Lamberg AL et al. Kidney disease and risk of venous thromboembolism: a nationwide population-based case-control study. J Thromb Haemost 2014;12:1449–54. https://doi.org/10.1111/jth.12652
6. Capodanno D, Angiolillo DJ. Antithrombotic therapy for atherosclerotic cardiovascular disease risk mitigation in patients with coronary artery disease and diabetes mellitus. Circulation 2020;142:2172–88. https://doi.org/10.1161/CIRCULATIONAHA.120.045465
7. Pecoits-Filho R, Abensur H, Betônico CCR et al. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr 2016;8:50. https://doi.org/10.1186/s13098-016-0159-z
8. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6:1246–58. https://doi.org/10.4239/wjd.v6.i13.1246
9. Usman MS, Khan MS, Butler J. The Interplay Between Diabetes, Cardiovascular Disease, and Kidney Disease. In: Chronic Kidney Disease and Type 2 Diabetes. Arlington (VA): American Diabetes Association. 2021. Available at: http://www.ncbi.nlm.nih.gov/books/NBK571718/ (accessed July 2024).
10. NICE. Cardiovascular disease: risk assessment and reduction, including lipid modification [CG181]. 2022. Available at: https://www.nice.org.uk/guidance/cg181 (accessed July 2024).
11. NICE. Clinical Knowledge Summary: CVD risk assessment and management. 2023. Available at: https://cks.nice.org.uk/topics/cvd-risk-assessment-management/ (accessed July 2024).
12. British Heart Foundation. UK Factsheet April 2023. 2023. Available at: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf?rev=e771367bf0654a4dae85cbc9dbefae17&hash=76C0182379BB6EE118EC6F76FA35A158 (accessed July 2024)
13. NHS England. NHS Long Term Plan. 2019. Available at: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed July 2024).
14. World Heart Federation. Hypertension. What we do. Available at: https://world-heart-federation.org/what-we-do/hypertension/ (accessed July 2024).
15. Natale P, Ni JY, Martinez-Martin D et al. Perspectives and experiences of self-monitoring of blood pressure among patients with hypertension: a systematic review of qualitative studies. Am J Hypertens 2023;36:372–84. https://doi.org/10.1093/ajh/hpad021
16. NHS. CVD PREVENT. Data & Improvement Tool. Available at: https://www.cvdprevent.nhs.uk/data-explorer?period=8 (accessed July 2024).
17. UCL Partners. Size of the prize – helping the NHS to prevent heart attacks and stroke at scale. UCLPartners. Available at: https://uclpartners.com/project/size-of-the-prize-for-preventing-heart-attacks-and-strokes-at-scale/ (accessed July 2024).
18. NHS England. Quality and Outcomes Framework guidance for 2023/24. Available at: https://www.england.nhs.uk/wp-content/uploads/2023/03/PRN00289-quality-and-outcomes-framework-guidance-for-2023-24.pdf (accessed July 2024).
19. NICE. CG76 Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. 2009. Available at: https://www.nice.org.uk/guidance/cg76 (accessed July 2024).
20. Waitzman E. Staff shortages in the NHS and social care sectors. 2022. Available at: https://lordslibrary.parliament.uk/staff-shortages-in-the-nhs-and-social-care-sectors/ (accessed July 2024).
21. Knuuti J, Wijns W, Saraste A et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. https://doi.org/10.1093/eurheartj/ehz425
22. Raleigh V, Jeffries D, Wellings D. Cardiovascular disease in England – supporting leaders to take actions. 2022. Available at: https://www.kingsfund.org.uk/sites/default/files/2022-11/CVD_Report_Web.pdf (accessed July 2024).
23. King’s Fund. Tackling cardiovascular disease: why the urgency? King’s Fund. 2022. Available at: https://www.kingsfund.org.uk/blog/2022/10/tackling-cardiovascular-disease-why-urgency (accessed July 2024).
24. UCL Partners. Proactive care frameworks. Available at: https://uclpartners.com/our-priorities/cardiovascular/proactive-care/ (accessed July 2024).
25. UCL Partners. Reducing the disease burden for hypertension in North East London. Available at: https://uclpartners.com/impact-story/reducing-the-disease-burden-for-hypertension-how-an-integrated-approach-is-improving-patient-care/ (accessed July 2024).
26. Jiang X, Ming W-K, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res 2019;21:e13166. https://doi.org/10.2196/13166
27. Bachtiger P, Petri CF, Scott FE et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health 2022;4:e117–25. https://doi.org/10.1016/S2589-7500(21)00256-9
28. Bachtiger P, Kelshiker MA, Petri CF et al. Survival and health economic outcomes in heart failure diagnosed at hospital admission versus community settings: a propensity-matched analysis. BMJ Health Care Inform 2023;30:e100718. https://doi.org/10.1136/bmjhci-2022-100718
29. NHS England. Personalised care. Available at: https://www.england.nhs.uk/personalisedcare/ (accessed July 2024).
30. Tucker KL, Sheppard JP, Stevens R et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med 2017;14:e1002389. https://doi.org/10.1371/journal.pmed.1002389
31. Steinsbekk A, Rygg LØ, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res 2012;12:213. https://doi.org/10.1186/1472-6963-12-213
32. MyRenalCare. Available at: https://www.myrenalcare.com (accessed July 2024).
33. Coulter A, Roberts S, Dixon. Delivering better services for people with long-term conditions. King’s Fund. 2013. Available at: https://www.kingsfund.org.uk/publications/delivering-better-services-people-long-term-conditions (accessed July 2024).
34. Jasemi M, Valizadeh L, Zamanzadeh V, Keogh B. A concept analysis of holistic care by hybrid model. Indian J Palliat Care 2017;23:71–80. https://doi.org/10.4103/0973-1075.197960
35. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30. https://doi.org/10.2174/1573399811666150421110316
36. Ski CF, Cartledge S, Foldager D et al. Integrated care in cardiovascular disease: a statement of the Association of Cardiovascular Nursing and Allied Professions of the European Society of Cardiology. Eur J Cardiovasc Nurs 2023;22:e39–46. https://doi.org/10.1093/eurjcn/zvad009
37. Zhang L, Ogungbemi A, Trippier S et al. Hub-and-spoke model for thrombectomy service in UK NHS practice. Clin Med Lond Engl 2021;21:e26–31. https://doi.org/10.7861/clinmed.2020-0579
38. Worthmann H, Winzer S, Schuppner R, Gumbinger C, Barlinn J. Telestroke networks for area-wide access to endovascular stroke treatment. Neurol Res Pract 2023;5:9. https://doi.org/10.1186/s42466-023-00237-9
39. Teo YH, Lim ICZY, Tseng FS et al. Predicting clinical outcomes in acute ischemic stroke patients undergoing endovascular thrombectomy with machine learning: a systematic review and meta-analysis. Clin Neuroradiol 2021;31:1121–30. https://doi.org/10.1007/s00062-020-00990-3
40. Ramsay AI, Ledger J, Tomini SM et al. Prehospital video triage of potential stroke patients in North Central London and East Kent: rapid mixed-methods service evaluation. Southampton (UK): National Institute for Health and Care Research. 2022. Available at: http://www.ncbi.nlm.nih.gov/books/NBK584528/ (accessed July 2024).
41. NHS England. Stroke – Getting It Right First Time – GIRFT. 2022. Available at: https://gettingitrightfirsttime.co.uk/medical_specialties/stroke/ (accessed July 2024).
42. Lin Huey-Juan, Wolf Philip A, Kelly-Hayes Margaret et al. Stroke severity in atrial fibrillation. Stroke 1996;27:1760–4. https://doi.org/10.1161/01.STR.27.10.1760
43. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. https://doi.org/10.7326/0003-4819-146-12-200706190-00007
44. NHS England. Quality and Outcomes Framework, 2022–23. NHS Digital. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2022–23 (accessed July 2024).
45. Health Innovation Network. Supporting the detection of atrial fibrillation and optimising treatment. Available at: https://thehealthinnovationnetwork.co.uk/case_studies/supporting-the-detection-of-atrial-fibrillation-and-optimising-treatment/ (accessed July 2024).
46. Health Innovation Network. Cardiovascular disease. Available at: https://thehealthinnovationnetwork.co.uk/programmes/cardiovascular-disease/ (accessed July 2024).
47. Health Innovation Oxford & Thames Valley. Better care for patients with heart failure. Available at: https://www.healthinnovationoxford.org/our-work/our-programmes/adopting-innovation/cardiovascular-disease/heart-failure/ (accessed July 2024).
48. Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab 2019;21:1668–79. https://doi.org/10.1111/dom.13724
49. Khunti K, Davies MJ. Clinical inertia – time to reappraise the terminology? Prim Care Diabetes 2017;11:105–6. https://doi.org/10.1016/j.pcd.2017.01.007
50. Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab 2019;10:2042018819844694. https://doi.org/10.1177/2042018819844694
51. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes 2010;4:203–7. https://doi.org/10.1016/j.pcd.2010.07.003
52. Milman T, Joundi RA, Alotaibi NM, Saposnik G. Clinical inertia in the pharmacological management of hypertension: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11121. https://doi.org/10.1097/MD.0000000000011121
53. Powell RE, Zaccardi F, Beebe C et al. Strategies for overcoming therapeutic inertia in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2021;23:2137–54. https://doi.org/10.1111/dom.14455
54. Medicines and Healthcare Products Regulatory Agency. Innovative Licensing and Access Pathway. GOV.UK. 2023. Available at: https://www.gov.uk/guidance/innovative-licensing-and-access-pathway (accessed July 2024).
55. Medicines and Healthcare Products Regulatory Agency. Apply for the early access to medicines scheme (EAMS). GOV.UK. 2023. Available at: https://www.gov.uk/guidance/apply-for-the-early-access-to-medicines-scheme-eams (accessed July 2024).
56. NHS England. NHS Accelerated Access Collaborative. Available at: https://www.england.nhs.uk/aac/ (accessed July 2024).
57. Medicines and Healthcare Products Regulatory Agency. Access Consortium. GOV.UK. 2023. Available at: https://www.gov.uk/guidance/access-consortium (accessed July 2024).
58. Medicines and Healthcare Products Regulatory Agency. International Recognition Procedure. GOV.UK. 2023. Available at: https://www.gov.uk/government/publications/international-recognition-procedure/international-recognition-procedure (accessed July 2024).
59. Royal College of Physicians. Benefiting from the ‘research effect’. RCP Lond. 2019. Available at: https://www.rcplondon.ac.uk/projects/outputs/benefiting-research-effect (accessed July 2024).
60. Ozdemir BA, Karthikesalingam A, Sinha S et al. Research activity and the association with mortality. PloS One 2015;10:e0118253. https://doi.org/10.1371/journal.pone.0118253
61. National Institute for Health and Care Research. Participant in Research Experience Survey (PRES) 2021/22. 2022. Available at: https://www.nihr.ac.uk/documents/participant-in-research-experience-survey-pres-202122/31768 (accessed July 2024).
62. Bower P, Grigoroglou C, Anselmi L et al. Is health research undertaken where the burden of disease is greatest? Observational study of geographical inequalities in recruitment to research in England 2013–2018. BMC Med 2020;18:133. https://doi.org/10.1186/s12916-020-01555-4
63. Zannad F, Alikhaani J, Alikhaani S, et al. Patient-reported outcome measures and patient engagement in heart failure clinical trials: multi-stakeholder perspectives. Eur J Heart Fail 2023;25:478–87. https://doi.org/10.1002/ejhf.2828
64. McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
65. Packer M, Anker SD, Butler J et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190
66. Anker SD, Butler J, Filippatos G et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038
67. Solomon SD, McMurray JJV, Claggett B et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–98. https://doi.org/10.1056/NEJMoa2206286
68. McEwan P, Darlington O, McMurray JJV et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 2020;22:2147–56. https://doi.org/10.1002/ejhf.1978
69. NICE. Dapagliflozin for treating chronic heart failure with reduced ejection fraction [TA679]. Available at: https://www.nice.org.uk/guidance/ta679 (accessed July 2024).
70. Medicines and Healthcare Products Regulatory Agency. MHRA guidance on the use of real-world data in clinical studies to support regulatory decisions. GOV.UK. Available at: https://www.gov.uk/government/publications/mhra-guidance-on-the-use-of-real-world-data-in-clinical-studies-to-support-regulatory-decisions/mhra-guidance-on-the-use-of-real-world-data-in-clinical-studies-to-support-regulatory-decisions (accessed July 2024).
71. Medicines and Healthcare Products Regulatory Agency. MHRA guideline on randomised controlled trials using real-world data to support regulatory decisions. GOV.UK. Available at: https://www.gov.uk/government/publications/mhra-guidance-on-the-use-of-real-world-data-in-clinical-studies-to-support-regulatory-decisions/mhra-guideline-on-randomised-controlled-trials-using-real-world-data-to-support-regulatory-decisions (accessed July 2024).
72. Wright FL, Cheema K, Goldacre R et al. Effects of the COVID-19 pandemic on secondary care for cardiovascular disease in the UK: an electronic health record analysis across three countries. Eur Heart J Qual Care Clin Outcomes 2023;9:377–88. https://doi.org/10.1093/ehjqcco/qcac077
73. Banerjee A, Chen S, Pasea L et al. Excess deaths in people with cardiovascular diseases during the COVID-19 pandemic. Eur J Prev Cardiol 2021;28:1599–609. https://doi.org/10.1093/eurjpc/zwaa155
74. Nanjo A, Evans H, Direk K, Hayward AC, Story A, Banerjee A. Prevalence, incidence, and outcomes across cardiovascular diseases in homeless individuals using national linked electronic health records. Eur Heart J 2020;41:4011–20. https://doi.org/10.1093/eurheartj/ehaa795
75. Banerjee A, Pasea L, Chung S-C et al. A population-based study of 92 clinically recognized risk factors for heart failure: co-occurrence, prognosis and preventive potential. Eur J Heart Fail 2022;24:466–80. https://doi.org/10.1002/ejhf.2417
76. Banerjee A, Dashtban A, Chen S et al. Identifying subtypes of heart failure from three electronic health record sources with machine learning: an external, prognostic, and genetic validation study. Lancet Digit Health 2023;5:e370–9. https://doi.org/10.1016/S2589-7500(23)00065-1
77. Dashtban A, Mizani MA, Pasea L et al. Identifying subtypes of chronic kidney disease with machine learning: development, internal validation and prognostic validation using linked electronic health records in 350,067 individuals. EBioMedicine 2023;89:104489. https://doi.org/10.1016/j.ebiom.2023.104489
78. Imperial College London. NCT05987670 Triple cardiovascular disease detection with an artificial intelligence-enabled stethoscope. 2023. Available at: https://clinicaltrials.gov/study/NCT05987670 (accessed July 2024).
79. NIHR Imperial Biomedical Research Centre. AI stethoscope helping diagnose heart failure is rolled out to 100 GP clinics. 2023. Available at: https://imperialbrc.nihr.ac.uk/2023/11/09/ai-stethoscope-helping-diagnose-heart-failure-is-rolled-out-to-100-gp-clinics/ (accessed July 2024).
80. UCL Partners. CVDACTION: Transforming the prevention of cardiovascular disease. Available at: https://uclpartners.com/our-priorities/cardiovascular/cvdaction-transforming-the-prevention-of-cardiovascular-disease/ (accessed July 2024).



