Genome-wide association and Mendelian randomisation studies have identified lipoprotein(a) (Lp[a]) as an emerging risk factor for calcific aortic stenosis and a causal risk factor for atherosclerotic cardiovascular disease (ASCVD) in different ethnicities. Given that Lp(a) is predominantly genetically determined, blood levels are relatively stable over time. The European Atherosclerosis Society recommends Lp(a) measurement in all adults at least once during their lifetime unless a secondary cause is suspected, or a specific treatment is instituted at lower levels. HEART UK recommends Lp(a) measurement in specific ‘at-risk’ cohorts. A new risk calculator introduced in association with the 2022 European Atherosclerosis Society guidelines calculates global ASCVD risk with and without Lp(a) concentrations alongside traditional risk factors; it highlights the importance of measuring Lp(a) in individuals to identify those with high or very high levels. Clinical laboratories therefore need to deliver timely, accurate and standardised measurements of Lp(a). The development of a reference material (WHO/IFCC SRM-2B) to assign molar values to calibrators alongside immunoassays that minimise sensitivity to the effect of isoform size (e.g., Denka reagent) offers a reliable and consistent assessment of graded Lp(a)-associated ASCVD risk based on particle number, which is endorsed by the European Atherosclerosis Society and HEART UK.
Why should lipoprotein(a) be measured?

The cardiovascular risk conferred by serum lipoprotein(a) (Lp(a)) in large noteworthy epidemiological studies1,2 over two decades ago was inconsistent and often underestimated owing to poor standardisation of the commercially available Lp(a) immunoassays. During the last decade, however, genome-wide association and Mendelian randomisation studies have identified Lp(a) as a new risk factor for calcific aortic stenosis and as a causal risk factor for atherosclerotic cardiovascular disease (ASCVD) across ethnicities.3,4 Elevated Lp(a) is associated with accelerated progression of low-attenuation plaque formation independent of traditional ASCVD risk factors,5 and Lp(a) drives plaque progression and vulnerability.6 Using data from UK Biobank, the 2022 Lp(a) European Atherosclerosis Society consensus reported that the relationship between Lp(a) concentration and ASCVD risk is continuous without any threshold effect: i.e., the higher the concentration, the higher the risk, which is observed across different ethnic groups.7 Over a median follow-up period of 11.2 years, the relationship between Lp(a) levels and ASCVD appear linear with hazard ratios of 1.11, 1.10 and 1.07 per 50 nmol/L increment for White, South Asian and Black individuals, respectively.8 Progress has been made in terms of identifying populations that are at risk of elevated Lp(a) and there are several phase II/III trials of Lp(a)-lowering therapies in progress. However, accurately measuring serum Lp(a) in a standardised way is progressing more slowly, which is at least partly due to its structure.
How should Lp(a) be measured?
Approximately 90% of plasma Lp(a) is genetically determined by polymorphisms of kringle IV (KIV) in the LPA gene.9 The repetitive structure of the KIV repeats is both clinically and analytically significant. Clinically, fewer numbers of KIV type-2 (KIV-2) repeats (i.e., <22) are associated with smaller apo(a) isoforms, which are more readily synthesised by the liver, resulting in higher circulating concentrations.10 Conversely, larger numbers of KIV-2 repeats increase the size of apo(a), resulting in reduced hepatic production and serum concentration.10 Analytically, the KIV-2 repeats present several challenges for the accurate measurement of Lp(a) by immunoassay. The antibodies raised to measure apo(a) are usually polyclonal and bind to the multiple KIV-2 repeats of apo(a). Depending on the calibrators used, this overestimates plasma Lp(a) concentrations in individuals with large isoforms and underestimates the concentrations in those with small isoforms.11 Some commercially available assays use Denka-based reagents (Denka Seiken Co. Ltd, Japan), which minimises isoform-dependent bias by using 5-point calibration consisting of separate pools of Lp(a) isoforms for varying concentrations.11 Indeed, a well-characterised reference material assigned in molar values (WHO/IFCC SRM-2B) is now available and can be used to assign molar values to immunoassay calibrators.12 Historically, plasma concentrations of Lp(a) were reported in mass units (mg/dL) but this is fundamentally unsound because well-designed immunoassays only measure apo(a) to reflect particle number.10 The implications of plasma Lp(a) results for risk assessment will vary depending on the units reported (i.e., 200 mg/dL, 200 mg/L and 200 nmol/L); therefore, an Lp(a) concentration without a unit cannot be interpreted. According to the European Atherosclerosis Society (EAS) consensus statement, the most appropriate unit of measurement for Lp(a) is nmol/L.13 Since the mass of Lp(a) is variable, converting between mass and molar units of Lp(a) is not recommended.10
In whom should Lp(a) be measured?
Table 1. Indications for lipoprotein(a) testing according to HEART UK (2019)14
| Indications for lipoprotein(a) testing |
|
|
|
|
|
The immunoassays available in clinical practice are not yet internationally standardised but they are most likely adequate for risk discrimination, i.e., most assays can readily identify individuals with high or very high Lp(a) concentrations relative to those with low or intermediate concentrations.13 Rather than absolute values, the EAS consensus statement (2022) suggests that clinical guidelines should consider using risk thresholds to either rule in (≥125 nmol/L) or rule out (<75 nmol/L) cardiovascular risk with a ‘grey’ zone (75–125 nmol/L).13 Table 1 highlights the populations in whom serum Lp(a) should be measured according to HEART UK.14 More recently, guidelines and consensus statements are moving to recommend Lp(a) measurement in all adults at least once during their lifetime and one approach to meet this universal screening recommendation is to include Lp(a) as part of initial lipid testing.13 Based on data from UK Biobank, it is clear that the risk for a cardiovascular event is underestimated substantially if Lp(a) is high.8 This is supported by a cross-sectional case-control study measuring traditional ASCVD risk factors in more than 12,000 individuals, which reported that 31–63% of individuals were reclassified from moderate to higher ASCVD risk with Lp(a) concentrations >99th percentile.15 Traditional ASCVD risk calculators do not include Lp(a); however, a new risk calculator introduced under the 2022 EAS consensus statement calculates global ASCVD risk with and without Lp(a) concentrations alongside traditional risk factors.13 Using this 2022 calculator, it is clear that the risk for a heart attack or stroke is underestimated substantially by traditional calculations that do not take account of elevated Lp(a).13 Furthermore, evidence from intervention trials in primary and secondary prevention settings revealed that Lp(a) is a risk factor even among statin-treated individuals with very low LDL-C concentrations16 and therefore, intensifying management of traditional ASCVD risk factors can mitigate at least part of the global risk of an individual, even if the Lp(a)-attributable risk is not modified.13 At present, there are no licensed Lp(a)-lowering pharmacotherapies; however, it is clear that measuring Lp(a) can personalise ASCVD risk and impacts the intensity of managing traditional risk factors.
In the UK and Ireland, the National Clinical Guideline for Stroke recommends that Lp(a) measurement should be considered in people below the age of 60 years with ischaemic stroke or transient ischaemic attack due to atherosclerosis, and Lp(a) levels ≥200 nmol/L should prompt specialist referral.17 According to the European guidelines, Lp(a) levels should also be measured in children in selected cases and in the context of family cascade testing13; in those with a history of ischaemic arterial stroke,10 although the association between Lp(a) and ischaemic arterial stroke is somewhat stronger if there are recurrent events18; and in those children of a parent with premature ASCVD and no other identifiable risk factors.13 Given that knowledge of Lp(a) can alter the management of other ASCVD risk factors, it is reasonable to measure Lp(a) in a child if their first-degree relative has markedly elevated Lp(a) concentrations using a systematic or opportunistic approach.14 For example, where services already exist for cascade testing for familial hypercholesterolaemia, these could be extended to cascade testing for elevated Lp(a).13,14 It should be noted that studies in familial hypercholesterolaemia found that although parents generally supported the idea of cascade testing for their children, some reported inappropriate information being shared with the children, or shared in too much detail, too quickly.19 If Lp(a) levels are measured in a child, it is important to openly discuss with the caregivers regarding the best way to communicate these findings with their child/children, what they mean for future risk, and treatment options.
In children, repeating Lp(a) testing may be required as levels can increase until adulthood. A Dutch study involving 2,740 children reported that mean Lp(a) levels increased from eight years of age, although this was less frequent in those who reached adulthood without lipid-lowering therapy than those subsequently treated with statins or ezetimibe (22% vs. 43%, respectively; 9% for those on ezetimibe).20
Although Lp(a) levels are genetically determined and therefore considered to be stable in adulthood, there are non-genetic factors that modify Lp(a) levels in children and adults; therefore, there are clinical situations in which Lp(a) may need to be measured more than once.13 Impaired kidney function may increase levels, possibly due to increased hepatic Lp(a) synthesis stimulated by loss of protein by proteinuria (i.e., nephrotic syndrome) or through peritoneal dialysate or due to reduced catabolism. Growth hormone replacement in patients with growth hormone deficiency as well as hypothyroidism may increase Lp(a) levels by twofold and therefore, repeating Lp(a) testing on correction of hormonal status is reasonable.13 HEART UK recommend that non-selective lipoprotein apheresis should be considered in patients with progressive coronary artery disease and Lp(a) levels greater than approximately 150 nmol/L whose LDL cholesterol remains >3.3 mmol/L, despite maximal lipid-lowering medications. Measuring Lp(a) following apheresis treatment is an important reason to retest this lipoprotein as it will identify the response to treatment.14
Case study
A 57-year-old non-smoking female was referred to the lipid clinic with an abnormal lipid profile and a background of cardiovascular disease; she had a past medical history of hypertension, dyslipidaemia, primary percutaneous coronary intervention to the left anterior descending artery (PCI-LAD) and fibromyalgia. In terms of family history, the father and brother both suffered a myocardial infarction at the ages of 59 and 62 years, respectively. There were no other risk factors for atherosclerotic cardiovascular disease (ASCVD).
Lipid profile
- Lipoprotein(a) 355 nmol/L
- Total cholesterol 4.8 mmol/L
- LDL-cholesterol 2.5 mmol/L
- HDL-cholesterol 1.4 mmol/L
- Triglycerides 1.8 mmol/L
- eGFR 85 mL/min/1.73 m2
- CRP 1 mg/L
- HbA1C 28 mmol/mol
Medications
- Lisinopril 10 mg once daily
- Atorvastatin 20 mg once daily
- Aspirin 75 mg once daily
- Clopidogrel 75 mg once daily
- Metoprolol 50 mg twice daily
Physical examination
- Blood pressure: 132/79 mmHg
- Height 1.79 m
- Weight 79 kg
- BMI 24.7 kg/m2
- Examination was unremarkable; important findings include the absence of xanthelasma or tendon xanthomata
The personal history of PCI-LAD in a non-smoker with a family history of myocardial infarction in two first-degree relatives suggests a genetic basis for this patient’s dyslipidaemia. Acknowledging that atorvastatin 20 mg once daily lowers LDL-cholesterol (LDL-C) by ~40%, it is unlikely that this patient’s lipid profile prior to atorvastatin 20 mg would exceed the LDL-C and total cholesterol thresholds in the Simon-Broome Criteria for familial hypercholesterolaemia (table 2).21 This presentation should therefore prompt the consideration of measuring lipoprotein(a) (Lp(a)) levels, which are predominantly (>90%) genetically determined with an autosomal co-dominant inheritance pattern.
Table 2. Simon-Broome criteria for diagnosis of familial hypercholesterolaemia21
| Definite familial hypercholesterolemia | |
| Elevated cholesterol Adult: Total cholesterol levels >290 mg/dL (7.5 mmol/L) or LDL-C >190 mg/dL (4.9 mmol/L) Child <16 years of age: Total cholesterol levels >260 mg/dL (6.7 mmol/L) or LDL-C >155 mg/dL (4.0 mmol/L) |
Plus one of: Tendon xanthomas in patient or in 1st degree relative (parent, sibling, child) or in 2nd degree relative (grandparent, uncle, aunt) Or DNA-based evidence of an LDL receptor mutation or familial defective apo B 100 or PCSK9 mutation |
| Possible familial hypercholesterolaemia | |
| Adult: Total cholesterol levels >290 mg/dL (7.5 mmol/L) or LDL-C >190 mg/dL (4.9 mmol/L) Child <16 years of age: Total cholesterol levels >260 mg/dL (6.7 mmol/L) or LDL-C >155 mg/dL (4.0 mmol/L) |
Plus one of: Family history of myocardial infarction: below age of 50 in 2nd degree relative or below age 60 in 1st degree relative Or Family history of raised cholesterol: >7.5 mmol/L in adult 1st or 2nd degree relative, or >6.7 mmol/L in child or sibling <16 years of age |
What if the clinician had known about the Lp(a) concentration before the cardiovascular event? One could assess this patient’s atherosclerotic cardiovascular disease risk (up to the age of 80 years) using the Lp(a) Clinical Guidance calculator, which was developed based on UK Biobank data.22 Without including Lp(a) in the risk assessment, this would have been underestimated at 17% but when Lp(a) is included in the calculation, this risk increased to 40%, as shown in figure 1.22 This underscores the importance of measuring Lp(a) in individuals with a family history of coronary artery disease. This patient’s risk of a heart attack or stroke could have been reduced to 23.1%, according to the Lp(a) Clinical Guidance calculator, if LDL-C and systolic blood pressure had been lowered prior to the event by 1.2 mmol/L and 10 mmHg, respectively (figure 2). Knowledge of Lp(a) therefore guides clinicians to aggressively treat traditional atherosclerotic risk factors in an attempt to reduce the Lp(a)-attributable risk. It also allows transparent communication with the patient regarding their level of cardiovascular risk, and therefore supports them in making informed decisions about their treatment options and lifestyle changes. Patients often find it difficult to understand and retain information regarding risk; the Lp(a) Clinical Guidance calculator can be shown to the patient to provide a visual aid of how changes in their Lp(a) level influence the risk of cardiovascular events.
![Cegla - Figure 1. Risk for a heart attack or stroke calculated up to 80 years of age using the Lp(a) Clinical Guidance calculator (http://www.lpaclinicalguidance.com/) with (red) and without (blue) incorporating lipoprotein(a) (Lp[a]) levels](https://bjcardio.co.uk/wp-content/uploads/2024/07/Cegla-Figure-1.png)
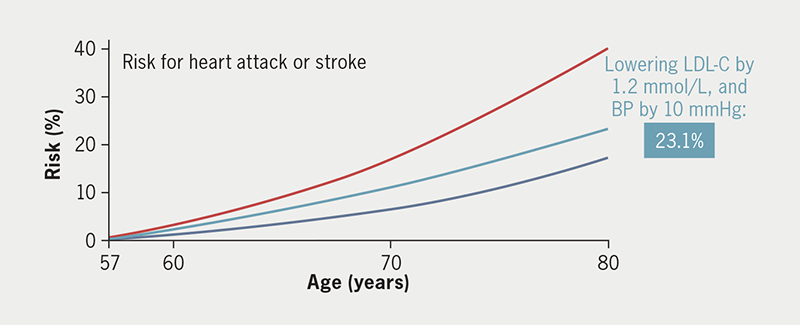
| Key: LDL-C = low-density lipoprotein cholesterol; BP = blood pressure (http://www.lpaclinicalguidance.com/) |
Given the family history of myocardial infarction in a family member <60 years old, cascade testing was performed. The patient’s family history co-segregated with Lp(a) (figure 3). A Lp(a) level of 255 nmol/L was found in the brother – which according to HEART UK – confers a high risk of cardiovascular disease,14 while an Lp(a) result ≥150 nmol/L is considered to ‘rule in’ cardiovascular risk by the European Atherosclerosis Society.13 Unfortunately, the patient’s father passed away before cascade testing was performed.
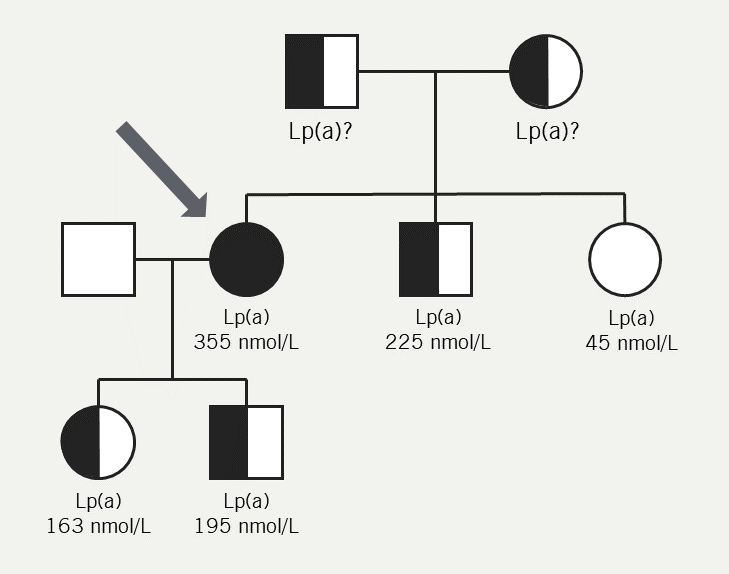
| Key: Lp(a) = lipoprotein(a) Figure adapted with permission from Cegla et al.14 |
Conclusion
The need for clinical laboratories to delivery timely, accurate and standardised measurements of Lp(a) is a growing area of high importance. A recent survey of laboratories within the UK found considerable variation in the analysis and reporting of Lp(a), for example in the use of mass or molar units, and in the reference ranges used.23 Further collaborative effort is needed to harmonise the reporting and interpretation of results. Expert working groups for the standardisation of Lp(a) measurement are progressing well but there is still some way to go. Their output will yield new reference methods for Lp(a) measurement and improve reference materials that are available to clinical assay manufacturers. In the next couple of years, these efforts will improve standardisation and harmonisation of Lp(a) assays. Clinically, knowledge of Lp(a) concentration contributes towards personalised medicine because global ASCVD risk may be underestimated substantially in individuals with unmeasured high or very high Lp(a) levels, and knowledge of the Lp(a) level directly impacts the intensity of risk factor management.
Key messages
- Lipoprotein(a) (Lp[a]) is genetically determined and the KIV polymorphism explains most of the variability in Lp(a) concentration and pathogeneticity in the population
- According to the European Atherosclerosis Society, Lp(a) measurement is recommended in all adults at least once during the lifetime unless a secondary cause is suspected, or a specific treatment is instituted to lower levels
- Laboratories should use an Lp(a) assay that is insensitive to apo(a) isoform size with a calibrator traceable to reference materials and reports results in nmol/L.
Conflicts of interest
JC has received lecture honoraria, consultancy fees, and/or research funding from Novartis, Silence Therapeutics and Amgen. JC and SA have each received an honorarium for their work on this supplement.
Saleem Ansari
Registrar in Metabolic Medicine and Chemical Pathology
Lipids and Cardiovascular Risk Service, Department of Cardiology, Hammersmith Hospital
Jaimini Cegla
Consultant in Metabolic Medicine and Chemical Pathology
Lipids and Cardiovascular Risk Service, Department of Cardiology, Hammersmith Hospital
Articles in this supplement
Introduction
Lipoprotein(a) in atherosclerotic heart disease and familial hypercholesterolaemia
Clinical utility of lipoprotein(a): an interventionist’s perspective
Raised lipoprotein(a): real-world examples of communication and clinical management
References
1. Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016;57:1953–75. https://doi.org/10.1194/jlr.R071233 [Epub online ahead of print]
2. Rifai N, Ma J, Sacks FM, Ridker PM et al. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: the Physicians’ Health Study. Clin Chem 2004;50:1364–71. https://doi.org/10.1373/clinchem.2003.030031 [Epub online ahead of print]
3. Coassin S, Kronenberg F. Lipoprotein(a) beyond the kringle IV repeat polymorphism: the complexity of genetic variation in the LPA gene. Atherosclerosis 2022;349:17–35. https://doi.org/10.1016/j.atherosclerosis.2022.04.003
4. Schunkert H, König IR, Kathiresan S et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011;43:333–8. https://doi.org/10.1038/ng.784
5. Kaiser Y, Daghem M, Tzolos E et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol 2022;79:223–33. https://doi.org/10.1016/j.jacc.2021.10.044
6. Kato A, Kinoshita D, Nagata T et al. Lipoprotein(a) levels and vulnerable characteristics in nonculprit plaque in patients with acute coronary syndrome. Int J Cardiol Heart Vasc 2022;43:101120. https://doi.org/10.1016/j.ijcha.2022.101120
7. Kronenberg F, Mora S, Stroes ESG et al. Frequent questions and responses on the 2022 lipoprotein(a) consensus statement of the European Atherosclerosis Society. Atherosclerosis 2023;374:107–20. https://doi.org/10.1016/j.atherosclerosis.2023.04.012 [Epub online ahead of print]
8. Patel AP, Wang M, Pirruccello JP et al. Lp(a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease new insights from a large national biobank. Arterioscler Thromb Vasc Biol 2021;41:465–74. https://doi.org/10.1161/ATVBAHA.120.315291 [Epub online ahead of print]
9. Scanu AM, Lawn RM, Berg K. Lipoprotein(a) and atherosclerosis. Ann Intern Med 1991;115:209–18. https://doi.org/10.7326/0003-4819-115-3-209
10. Cegla J, France M, Marcovina SM, Neely RDG. Lp(a): When and how to measure it. Ann Clin Biochem 2021;58:16–21. https://doi.org/10.1177/0004563220968473 [Epub online ahead of print]
11. Boot CS. How to measure lipoprotein(a) and in whom? Br J Cardiol 2022;29(suppl 1):S15–S19. https://doi.org/10.5837/bjc.2022.s04
12. Dati F, Tate JR, Marcovina SM, Steinmetz A; International Federation of Clinical Chemistry and Laboratory Medicine; IFCC Working Group for Lipoprotein(a) Assay Standardization. First WHO/IFCC international reference reagent for lipoprotein(a) for immunoassay–Lp(a) SRM 2B. Clin Chem Lab Med 2004;42:670–6. https://doi.org/10.1515/CCLM.2004.114
13. Kronenberg F, Mora S, Stroes ESG et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J 2022;43:3925–46. https://doi.org/10.1093/eurheartj/ehac361
14. Cegla J, Neely RDG, France M et al. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. https://doi.org/10.1016/j.atherosclerosis.2019.10.011 [Epub online ahead of print]
15. Nurmohamed NS, Kaiser Y, Schuitema PCE et al. Finding very high lipoprotein(a): the need for routine assessment. Eur J Prev Cardiol 2022;29:769–76. https://doi.org/10.1093/eurjpc/zwab16718
16. Willeit P, Ridker PM, Nestel PJ et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 2018;392:1311–20. https://doi.org/10.1016/S0140-6736(18)31652-0 [Epub online ahead of print]
17. National Clinical Guideline for Stroke for the UK and Ireland. London: Intercollegiate Stroke Working Party; 2023 May 4. Available at: www.strokeguideline.org (accessed February 2024)
18. Sultan SM, Schupf N, Dowling MM, Deveber GA, Kirton A, Elkind MSV. Review of lipid and lipoprotein(a) abnormalities in childhood arterial ischemic stroke. Int J Stroke 2014;9:79–87. https://doi.org/10.1111/ijs.12136 [Epub online ahead of print]
19. Keenan KF, Finnie RM, Simpson WG, McKee L, Dean J, Miedzybrodzka Z. Parents’ views of genetic testing and treatment of familial hypercholesterolaemia in children: a qualitative study. J Community Genet 2019;10:129–41. https://doi.org/10.1007/s12687-018-0373-5 [Epub online]
20. de Boer LM, Hof MH, Wiegman A, Stroobants AK, Kastelein JJP, Hutten BA. Lipoprotein(a) levels from childhood to adulthood: data in nearly 3,000 children who visited a pediatric lipid clinic. Atherosclerosis 2022;349:227–32. https://doi.org/10.1016/j.atherosclerosis.2022.03.004 [Epub online ahead of print]
21. Marks D, Thorogood M, Neil HAW, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis 2003;168:1–14. https://doi.org/10.1016/s0021-9150(02)00330-1
22. Lp(a) Clinical Guidance. 2023. Available at: https://www.lpaclinicalguidance.com/ (accessed February 2024)
23. Ansari S, Neely RDG, Payne J, Cegla J. The current status of lipoprotein(a) measurement in clinical biochemistry laboratories in the UK: Results of a 2021 national survey. Ann Clin Biochem 2024;61:195–203. https://doi.org/10.1177/00045632231210682
