Though a rare condition, acute type A aortic dissection (ATAAD) is associated with high morbidity and mortality; hence, timely diagnosis and surgery are important to reduce the risk of mortality. If the dissection extends into the aortic arch branches, ensuring adequate cerebral perfusion during surgery is crucial to preventing stroke.
A 50-year-old man presented to the emergency department with symptoms of acute chest pain, dizziness, and headache. His blood pressure was 180/110 mmHg and heart rate was 100 bpm. He had a high blood pressure and heart rate and was initially treated with glyceryl trinitrate. Initial investigations ruled out acute coronary syndrome. Further investigations revealed ATAAD with the involvement of arch branches, an incomplete (open) circle of Willis and cerebral malperfusion. He was prepared for a branch-first total aortic arch replacement. Due to the high risk of stroke in this patient, off-pump axillo-axillary bypass (adaptive perfusion technique) was used to ensure bihemispheric cerebral perfusion throughout the surgery. The surgery was uneventful, and the patient was discharged 12 days later. Postoperative follow-up at six months was normal.
In conclusion, ATAAD is a surgical emergency that can mimic other acute thoracic conditions, such as pulmonary embolism and acute coronary syndrome; therefore, a judicious approach should be applied in the diagnosis and early management of symptoms. The reconstruction technique should be tailored to the patient’s needs; as this patient had a poorly functioning circle of Willis and cerebral hypoperfusion, continuous bilateral cerebral perfusion was essential to prevent irreversible cerebral ischaemic insult. The adaptive technique is easy to learn for surgeons who are already proficient in aortic dissection procedures, it is reproducible and requires only minor changes to the surgical setup without any substantial increase in operative time. Adoption of this technique in other surgical centres could be beneficial in increasing the success rate for the treatment of ATAAD.
Introduction
Acute type A aortic dissection (ATAAD) complicated by cerebral malperfusion is a life-threatening condition that is associated with high morbidity and mortality.1 There is no consensus on which condition should be addressed first: the dissection or cerebral malperfusion.1 Severe brain injury at presentation has historically been seen as a reason to avoid emergency surgery, with several studies suggesting a delayed surgical approach until the patient’s neurological status improves.2 At the International Registry of Acute Aortic Dissection (IRAD) enrolling centres, the presence and type of brain injury significantly influenced patient management decisions. Surgery was not performed in 24% of patients with cerebrovascular accident (CVA) and 33% of patients in a coma, compared with only 11% of patients without brain injury. However, when examining hospital outcomes based on the type of therapeutic management, researchers found that medical therapy resulted in very poor outcomes: 100% mortality in coma patients and 76.2% in those with CVA. In contrast, surgery was shown to reduce mortality risk significantly (odds ratio [OR] 0.058, p<0.001), providing a 50% survival advantage over medical management.3 The gold standard of care remains controversial, and several issues remain under debate in the management of patients with ATAAD because of the difference in time from diagnosis to surgery, the extent of dissection and degree of malperfusion; hence, mostly every case of ATAAD requires a tailored approach. IRAD recommends that ATAAD patients with cerebral hypoperfusion should always be considered for surgical intervention, especially if early surgery is feasible and the cerebral hypoperfusion is not yet symptomatic.2 ATAAD-associated cerebral malperfusion is often due to the extension of the dissection into the aortic arch branches with or without thrombosis of these vessels.
This is a case report of a 50-year-old man who presented with symptoms of cerebral malperfusion and was later diagnosed with ATAAD with involvement of the arch branches. He was treated using the branch-first total aortic arch replacement (TAR). The major drawbacks of present methods of TAR are that debranching and reattachment of the arch branches are performed with on-pump cerebral perfusion; although this process mimics the normal physiology of brain blood supply, it involves the use of hypothermia, the absence of pulsatile cerebral blood flow, a different perfusion velocity, and periods of unilateral cerebral perfusion, which all lead to disruption of cerebral autoregulation of blood flow and increase the risks of neurological complications.4,5 Therefore, a new method called ‘adaptive perfusion technique’ was used to ensure continuous bihemispheric cerebral perfusion during the entire surgery.6
Case presentation
A 50-year-old man with a history of poorly controlled hypertension presented to the emergency department of a community hospital with sudden-onset chest pain, dyspnoea, generalised weakness, dizziness, headache, amnesia, and nausea. He denied previous episodes of chest pain. His recorded blood pressure (BP) was 180/110 mmHg, and heart rate (HR) was 100 bpm. He was treated with sublingual glyceryl trinitrate (0.6 mg) for angina, with minimal effect, while cardiac biomarkers (troponin and creatine kinase-myocardial band), an electrocardiogram (ECG) and a chest X-ray were ordered. The biomarkers and ECG were both normal, but the chest X-ray showed mediastinal widening. Intravenous labetalol was administered to keep his HR at 60–65 bpm and BP at 120/80 mmHg, in order to reduce the shearing force on the aortic wall.
Both transthoracic echocardiography (TTE) and contrast computed tomography (CT) angiography revealed a type A aortic dissection with involvement of the arch branches; dissection of the brachiocephalic trunk with thrombosis of the right common carotid artery (RCCA) and right subclavian artery, dissection and thrombosis of the left common carotid artery (LCCA) and left subclavian artery as depicted in figures 1A and 1B. A Matas test revealed an incomplete/open circle of Willis with inadequacy of collateral cerebral perfusion as indicated by red arrows in figures 1C and 1D.
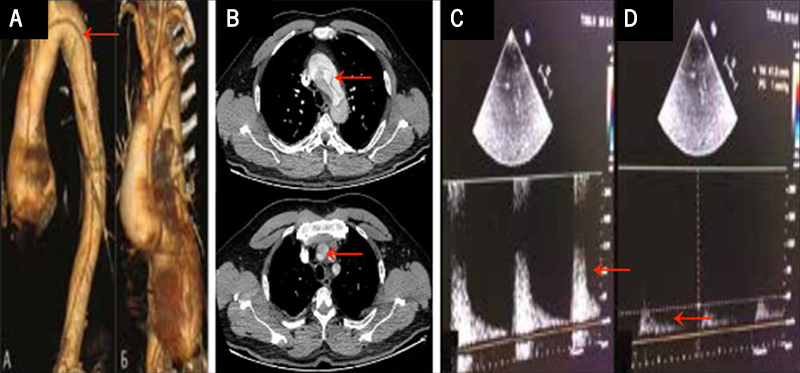
He was referred to the Bakulev Cardiovascular Center for a surgical treatment. On admission, his BP was 150/90 mmHg, HR was 90 bpm with normal rhythm, respiratory rate was 18 per min, oxygen saturation (SpO2) was 97%, and body temperature was 37oC. Cardiac examination revealed an early 2/6 diastolic murmur, best heard at the right second intercostal space; otherwise, all other physical examinations were non-contributory.
The patient had no history of heart or connective tissue disease. A repeat TTE revealed dissection of the aortic root and ascending aorta with an intimal flap and a grade 2 aortic regurgitation. The patient was prepared for surgery an hour later.
Surgery
Due to this patient’s cerebral malperfusion (CM) as a result of arch branches involvement and a poorly functioning circle of Willis, maintaining continuous bilateral-bihemispheric cerebral blood flow during reconstruction of the arch branches was essential to prevent a stroke.
The surgeons at our centre devised the ‘adaptive perfusion technique’, which involves the creation of a shunt between the right and left axillary arteries (RAA and LAA) to allow undisrupted bihemispheric cerebral perfusion for the entire duration of the branch-first TAR. The technique also includes a standby head pump connected to the perfusion circuit to augment the functioning of the shunt, in case cardiac contraction is not enough to maintain adequate antegrade cerebral perfusion during the branch-first stage.
To create the perfusion circuit, the axillary arteries were exposed in the infraclavicular fossa and mobilised. After median sternotomy, complete access to the ascending aorta, the aortic arch and its branches, and the part of the descending aorta for the distal anastomosis, is gained by their careful dissection from surrounding tissue. The aortic root was 55 mm, the ascending aorta 80 mm, and aortic arch 45 mm (figure 2A, black arrow showing aortic enlargement).
A single intravenous bolus of heparin (600 U/kg) was administered, and both axillary arteries were cannulated and connected to each other to form the RAA–LAA shunt portion of the circuit. The shunt was then connected to both the cardiopulmonary bypass (CPB) pump and separate head pump using arterial cannulae to complete the circuit (figure 2B).
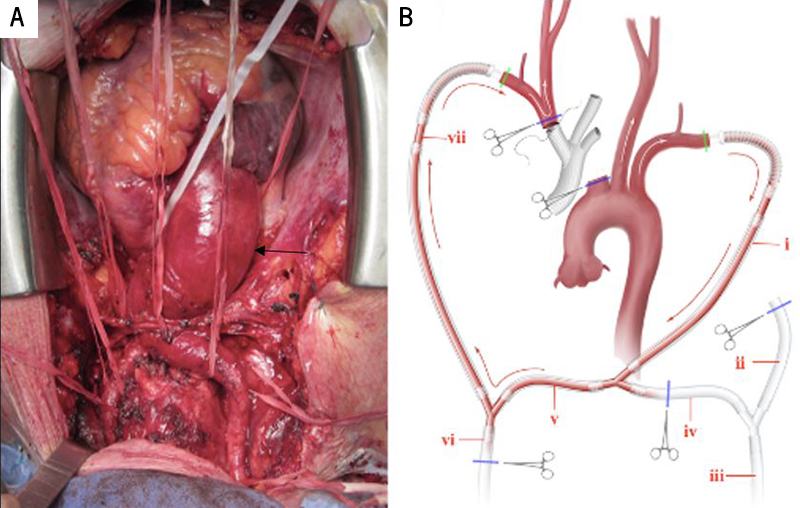
The inferior and superior vena cava (IVC and SVC) were also cannulated, and the left heart was vented through the right superior pulmonary vein. Though the CPB setup was complete, it was not in use during the branch-first stage of the reconstruction. The aortic arch branches were resected and anastomosed to a trifurcation graft (Bakulev Scientific Center of Cardiovascular Surgery, Moscow, Russia) in a sequential manner, starting from the brachiocephalic trunk to the left subclavian artery. The shunt ensured cerebral perfusion by passively (off-pump) transfusing blood from LAA to RAA and then to the right subclavian artery, brachiocephalic trunk, and left common carotid artery (LCCA) during the branch-first stage. If cardiac contraction had been inadequate during this stage of the procedure, the CPB or head pump could be started to augment the work of the shunt (a switch from off-pump to on-pump). This would help maintain the blood flow along the LCCA at a mean pressure of 55 mmHg and a velocity of 600 ml/min (figure 3A).
The adaptive perfusion circuit in the on-pump regimen also served as a source of systemic perfusion during the reconstruction of the ascending aorta and the aortic arch. The CPB and/or head pump supplied blood to the visceral organs through the LAA and the RAA. After debranching and anastomosis of the left subclavian artery to the trifurcation graft, CPB was started, and the patient was cooled to 26oC (to minimise the metabolic rate) for reconstruction of the ascending aorta and aortic arch using the ‘frozen elephant trunk technique’ (figure 3B). After completion of the distal aortic anastomosis using the Basex tube graft (Bakulev Scientific Center of Cardiovascular Surgery, Moscow, Russia), systemic perfusion was achieved through the side port of the tube graft while the proximal anastomosis to the aortic root was performed. The common stem of the trifurcation graft was passed under the innominate vein and anastomosed to the tube graft in an end-to-side fashion.
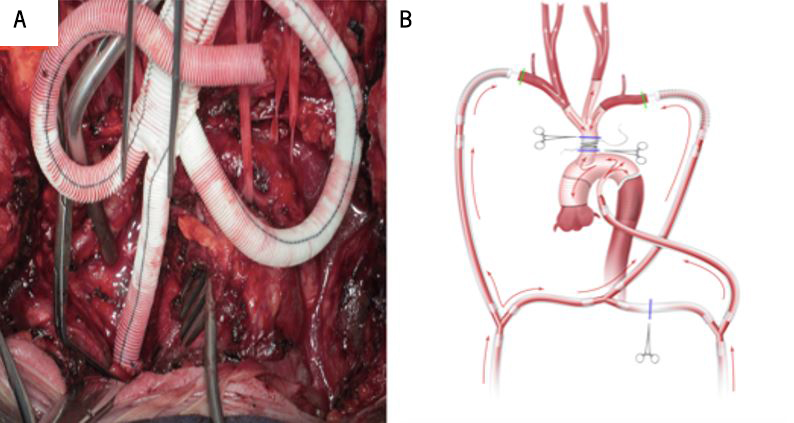
The adaptive perfusion technique ensured off-pump continuous bilateral cerebral perfusion along the carotid and/or vertebral arteries during the branch-first stage and on-pump whole-body perfusion during reconstruction of the ascending aorta and aortic arch. Cerebral perfusion was monitored using cerebral oximetry, an electroencephalogram bispectral index monitoring system, and a transcranial Doppler. Aortic regurgitation was insignificant after the replacement of the ascending aorta and aortic arch. A repeat CT angiography showed a well-reconstructed and functioning ascending aorta, aortic arch and its branches, shown by the red arrows in figures 4A and 4B.
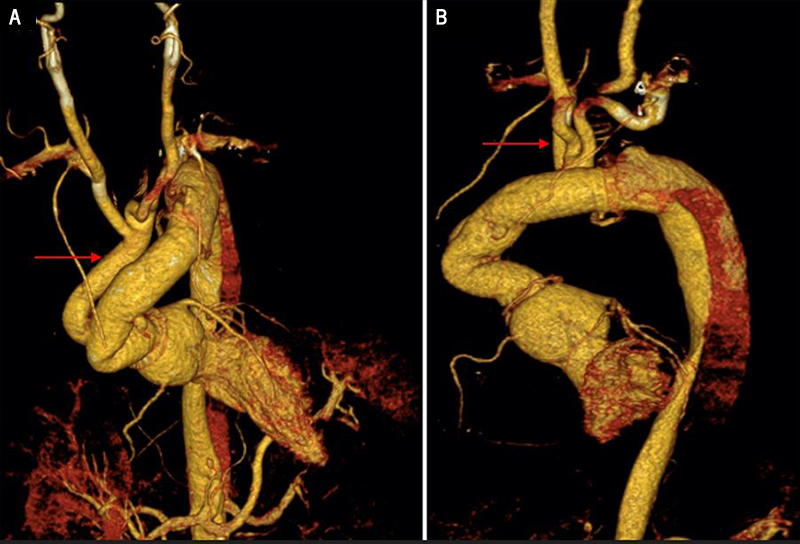
Discussion
ATAAD is a surgical emergency with operative mortality of about 30%, a rate that has not changed meaningfully in more than two decades.5 Mortality rates of medically managed cases are 20% at 24 hours after presentation, 30% at 48 hours, 40% at a week, and 50% at a month.6 Patients with ATAAD complicated by malperfusion syndrome represent one of the highest surgical risk groups.1 Malperfusion syndrome in ATAAD can affect any vascular bed and may lead to consequential end-organ ischaemia, such as myocardial, cerebral, spinal cord, visceral, and/or limb infarction.1 In an IRAD study of 2,402 patients undergoing surgical repair of ATAAD, 15.1% presented with CM and neurologic deficits.8 Compared with patients with normal cerebral perfusion, patients with CM had an increased incidence of postoperative cerebrovascular accidents (17.5% vs. 7.2%, p<0.001), acute kidney injury (28.3% vs. 18.1%, p<0.001) and in-hospital mortality (25.7% vs. 12.0%, p<0.001).9 That is why the branch-first technique is used in such cases, as it permits total aortic arch repair without global cerebral circulatory arrest, excessive hypothermia, extended periods of CPB, or cardiac and other organ circulatory exclusion.10 It also shortens distal organ and cardiac ischaemic time and reduces the opportunity for air and particulate embolisation during aortic repair.11 The adaptive perfusion technique protects the brain better against ischaemia because it keeps bihemispheric perfusion going while the repair process is going on. This technique, in contrast to traditional techniques, reduces the duration of hypothermia, pulseless cerebral blood-flow, and avoids unilateral cerebral perfusion, thereby minimising disruption of cerebral autoregulation of blood flow and the risks of neurological complications.
Early diagnosis and management of these ATAAD patients is crucial for optimal surgical outcome. Factors, such as patients’ age, clinical status at presentation, frailty, comorbidities, end-organ ischaemia, the anatomic location of the intimal tear, and the surgeon’s experience, influence operative outcomes.
Though urgent repair of ATAAD prevents death from aortic rupture, organ malperfusion, or acute aortic valve insufficiency, it is associated with the risk of haemorrhage due to anticoagulant use during CPB.12,13 This is a case report of a male patient who developed ATAAD with involvement of the arch branches and CM. He had no history of genetic disorders or connective tissue diseases. He was misdiagnosed with acute coronary syndrome because of his presentation: sudden onset of chest pain, dyspnoea, weakness, dizziness, cough, and neurological symptoms. After an ECG and cardiac biomarkers showed no abnormalities, a different diagnosis was sought.
Unlike in this case report, ATAAD is usually accompanied by symptoms of acute myocardial infarction, severe aortic valve insufficiency, and associated heart failure. Maintaining a high suspicion of ATAAD in patients with acute chest pain is important as it can masquerade as acute coronary syndrome.
Conclusion
ATAAD with CM is associated with high morbidity and mortality; hence, urgent surgical intervention is warranted. In most cases, branch-first TAR with reconstruction of the arch branches remains the preferred method of treatment. During the operation, we observed bihemispheric perfusion in the operating room as shown by the free blood flow into the brachiocephalic trunk and left carotid artery. Our approach (branch-first TAR with adaptive perfusion) ensures off-pump and on-pump continuous antegrade bihemispheric cerebral perfusion during the reconstruction of the arch branches and ascending aorta, respectively; this could potentially be especially useful to patients with a high risk of stroke (due to an incomplete circle of Willis in this case). Though this approach of cerebral perfusion is reproducible and copyable, further studies in a larger patient cohort are needed for its perfection and standardisation. Adoption of this technique in other surgical centres could be beneficial in increasing the success rate for the treatment of ATAAD. This technique could potentially improve postoperative morbidity and mortality significantly especially in ATAAD with high risk of stroke.
Conflicts of interest
None declared.
Funding
None.
Patient consent
The patient gave a verbal agreement for his condition and method of treatment to be published as a case report. A written agreement from patients for the sake of a case report is usually not required by the Institution’s Review Board.
Acknowledgement
A.N. Bakulev National Medical Research Centre of Cardiovascular Surgery and RUDN University.
References
1. Berretta P, Trimarchi S, Patel HJ, Gleason TG, Eagle KA, Di Eusanio M. Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD. J Vis Surg 2018;4:65. https://doi.org/10.21037/jovs.2018.03.13
2. Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr FW. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg 2009;138:1363–9. https://doi.org/10.1016/j.jtcvs.2009.04.059
3. Ahmed Y, van Bakel PAJ, Patel HJ. Addressing malperfusion first before repairing type A dissection. JTCVS Tech 2021;10:1–5. https://doi.org/10.1016/j.xjtc.2021.04.029
4. Sidiki AI, Faybushevich AG, Lishchuk AN, Koltunov AN, Roshchina EA. The Carpentier-Edwards classic and physio annuloplasty rings in repair of degenerative mitral valve disease: a retrospective study. J Saudi Heart Assoc 2020;32:224–32. https://doi.org/10.37616/2212-5043.1027
5. Lishchuk AN, Sidiki AI, Faybushevich AG, Ivanov DV, Roshchina EA. Application of autopericardium in mitral valve repair. Journal of New Medical Technologies 2019;26:24–8. https://doi.org/10.24411/1609-2163-2019-16523
6. Sabe AA, Percy ED, Kaneko T, Plichta RP, Hughes GC. When to consider deferral of surgery in acute type A aortic dissection: a review. Ann Thorac Surg 2021;111:1754–62. https://doi.org/10.1016/j.athoracsur.2020.08.002
7. Pacini D, Murana G, Di Marco L et al. Cerebral perfusion issues in type A aortic dissection. J Vis Surg 2018;4:77. https://doi.org/10.21037/jovs.2018.03.20
8. Sultan I, Bianco V, Patel HJ et al. Surgery for type A aortic dissection in patients with cerebral malperfusion: results from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg 2021;161:1713.e1–1720.e1. https://doi.org/10.1016/j.jtcvs.2019.11.003
9. Lou X, Chen EP. Malperfusion syndromes in acute type A aortic dissection. Vessel Plus 2022;6:38. https://doi.org/10.20517/2574-1209.2021.108
10. Matalanis G, Galvin SD. “Branch-first” continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection. Ann Cardiothorac Surg 2013;2:194–201. https://doi.org/10.3978/j.issn.2225-319X.2013.02.01
11. Kim M, Matalanis G. Technique and rationale for branch-first total aortic arch repair. JTCVS Tech 2020;4:1–4. https://doi.org/10.1016/j.xjtc.2020.09.014
12. Sidiki AI, Akulova AA, Hussein MH et al. Physio and Physio II rings: beyond the annular physiology. J Cardiovasc Surg (Torino) 2022;63:529–35. https://doi.org/10.23736/S0021-9509.22.11874-4
13. Wang C, Zhang L, Li T, Xi Z, Wu H, Li D. Surgical treatment of type A acute aortic dissection with cerebral malperfusion: a systematic review. J Cardiothorac Surg 2022;17:140. https://doi.org/10.1186/s13019-022-01894-8
