Heart failure (HF) is common and the current gold-standard diagnostic modality for left ventricular systolic dysfunction (LVSD) is transthoracic echocardiography (TTE). To comply with the National Service Framework (NSF) for Coronary Heart Disease, an open access TTE service was established and this paper reports on the diagnostic yield of LVSD and valvopathy of TTE services in that service.
Diagnostic services were made available to patients from both primary and secondary care. As part of the assessment, all patients were evaluated by TTE to assess left ventricular function and any valvular pathology. Overall, 61% of patients had normal left ventricular ejection fraction, 16% mild LVSD, 9% moderate LVSD and 14% severe LVSD. Forty-three per cent of patients had no evidence of valvopathy, 31% had mild, 19% moderate and 7% severe valvopathy. Valvopathy was the primary pathology in 15.8% of patients and 13.5% had LVSD as their primary pathology: 30.4% had no valvopathy or LVSD. In the remainder, it was not possible to determine the dominant pathology causing HF due to concomitant LVSD and valvopathy.
TTE has a very high diagnostic yield in both primary and secondary care. Significant levels of valvopathy and LVSD are found in populations from both primary and secondary care.
Introduction
Heart failure (HF) is common and is associated with a high morbidity and mortality. Forty per cent of patients with symptomatic left ventricular systolic dysfunction (LVSD) die within a year of diagnosis and 10% per annum thereafter, giving a five-year mortality rate of up to 70%.1 Estimates of the prevalence of symptomatic HF in the general European population range from 0.4–2%,2-4 with half of these patients suffering from LVSD and half from left ventricular diastolic dysfunction (LVDD).3,5 HF consumes nearly 2% of National Health Service (NHS) resources (a figure which will inevitably increase with the advent of relatively expensive device therapy, an ageing population and improved survival following myocardial infarction).6,7 In secondary care, 5% of hospital in-patients have a confirmed diagnosis of HF and account for 2% of NHS in-patient days. In
primary care, these patients have an average of 11 consultations per annum with their general practitioner (GP).
The most common cause of chronic HF is ischaemic heart disease.5 Transthoracic echocardiography (TTE) is considered one of the most sensitive, specific, easily performed and reproducible tools for diagnosing the aetiology and severity of LVSD.8 Delayed diagnosis and initiation of treatment in HF makes a difference to morbidity and mortality.9,10 There is evidence that many patients in the UK are either being treated suboptimally or not at all.11,12 Conversely, up to 50% of identified HF patients in primary care have no evidence of LVSD.13,14 Open access TTE services have been established in several British and European centres to improve this situation (see editorial on page 6).14 The National Service Framework (NSF) 2000 for Coronary Heart Disease15 has devoted an entire chapter to HF diagnostic and treatment services. Open access TTE services were established in Birmingham in 1993 and recently restructured to meet these NSF requirements. This paper reports on the results of these clinics to assess the impact and value of such NSF-structured facilities, which also serve to accommodate delivery of the published guidance on HF management from the National Institute for Health and Clinical Excellence (NICE).16
Methods
Service set-up
The open access HF service was expanded to provide GPs and hospital physicians a one-stop service for diagnosis and management advice for patients suspected to have HF. The expansion restructured one of the largest and longest running services of its kind in the UK. The primary and secondary care services in operation serve a population of approximately 330,000. The service operates on three sites, all of which are supported by specialist HF nurses. In primary care (two sites), all referred patients were assessed and received a TTE from a GP with a specialist interest (GPSI) in cardiology. In secondary care, all referrals were assessed by a physician (consultant cardiologist or specialist registrar) and received a 12-lead electrocardiogram (ECG), chest radiograph and TTE in a dedicated HF clinic. If indicated, further management and treatment optimisation occurred either in nurse-led HF clinics (on all sites) or secondary care HF clinics. In addition, the secondary care centre provided dedicated TTE for the assessment of hospital in-patients with acute coronary syndromes or suspected HF.
Patient cohort
Patients therefore came from four sources, and these were audited between January and September of 2004. First, those referred by their GP to the open-access HF clinic in primary care (PC cohort). Second, those referred by their GP to the open-access HF clinic in secondary care (SCP cohort). Third, patients with symptoms or signs of HF on in-patient medical wards (IP cohort). Finally, patients on the coronary care unit (CCU cohort), from where nurses on a daily basis activated referral of patients with an acute coronary syndrome or symptoms and/or signs of HF. For referrals from primary care, GPs were free to refer either to the primary care-based or secondary care-based service, with no deferential criteria for referral.
Transthoracic echocardiography
All patients had a TTE performed by a technician, GPwSI, specialist registrar or consultant cardiologist. An ejection fraction ≥50% defined normal systolic function, 40–49% mild LVSD, 30–39% moderate LVSD and ≤30% severe LVSD. Where possible, ejection fraction was measured but where this was not possible for technical reasons, the ejection fraction range was estimated by at least two experienced operators. Valvopathy was divided into mild, moderate or severe in accordance with conventional echocardiographic criteria. All cases were reviewed by a clinician.
Statistical analysis
All statistical analysis was done with Excel 2000 for Windows XP.
Results
Activity
Over the audited nine-month period, 703 patients underwent TTE. Seventy-one per cent of referrals came from primary care (29% were seen in the primary care clinic [PC] and 42% in the secondary care clinic [SCP]). Fourteen per cent of referrals were from the CCU cohort and 15% from the IP cohort (figure 1A). In SCP, 95% of consultations were new referrals and 5% follow-up consultations. In PC, all consultations were new referrals. The majority of follow-up activity occurred in the nurse-led HF clinics running in parallel to physician-led clinics in primary and secondary care. Sixty-one per cent of nurse-led consultations were in the secondary care clinic and the remainder in the primary care clinic (figure 1B). Reasons for consultation in the nurse-led clinics were almost identical in both settings, with approximately half of the overall consultations for drug initiation and up-titration (in particular beta blockers and angiotensin-converting enzyme [ACE] inhibitors) and half for patient review (figures 1C and 1D).
Review of left ventricular function (table 1)
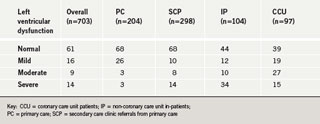
Overall, 61% of patients had normal left ventricular function, 16% mild LVSD, 9% moderate LVSD and 14% severe LVSD. However, the distribution of LVSD differed according to source. The PC and SCP cohorts had an identical prevalence of normal left ventricular function (68%). Of the remaining 32% of patients, in those referred to SCP, more had moderate and severe LVSD than those referred to PC. Not unexpectedly, the lowest prevalence of normal left ventricular function (39%) in any group was found in CCU patients: 19% of CCU patients had mild LVSD, 27% moderate LVSD and 15% severe LVSD. In the IP group the incidence of severe LVSD was the highest of any group at 34%, while levels of mild and moderate LVSD were slightly lower than the CCU group at 12% and 10%, respectively.
Review of valvopathy (figure 2)
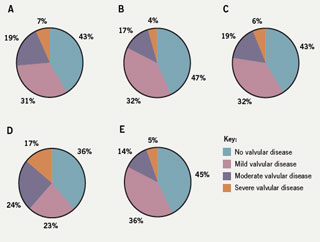
Overall, 43% of patients had no evidence of valvopathy: 31% had mild, 19% moderate and 7% severe valvopathy. The distribution of valvopathy was almost identical in the out-patient populations referred to either PC or SCP. Likewise, CCU patients were very similar and showed a distribution of valvopathy comparable with out-patients. Review of the IP group revealed the highest prevalence of valvopathy of any of the groups. Only 36% of this group had no significant valvopathy. There was a striking 17% incidence of severe valvopathy, which could significantly contribute to symptoms or signs of HF in the absence of LVSD. Twenty-three per cent of this cohort had mild valvopathy and 24% moderate valvopathy.
Other findings
TTE also revealed other structural and functional abnormalities during echocardiography. There were a significant number of ‘other’ diagnoses including left ventricular hypertrophy (LVH), pericardial effusion, left ventricular thrombus (LVTh) and pulmonary hypertension (PHT). Of the IP group, 22% were shown to have LVH and 26% PHT. In the CCU group, 22% had LVH and a striking 10% were shown to have LVTh. Patients referred by GPs to either PC or SCP showed a 17% incidence of LVH and 13% incidence of PHT. One patient from primary care was also shown to have an atrial myxoma.
LVSD versus valvopathy as the primary pathology
, n=703)”]![Table 2. Is left ventricular systolic dysfunction or valvular disease the primary pathology? (Values reflect absolute number of patients [%], n=703) Table 2. Is left ventricular systolic dysfunction or valvular disease the primary pathology? (Values reflect absolute number of patients [%], n=703)](https://bjcardio.co.uk/files/uploads/2008/01/Br-J-Cardiol-2008-15-35-39-table-2.jpg) Table 2 illustrates that nearly a third (214, 30.4%) of all patients had no LVSD and no valvopathy. Of the patients with normal left ventricular function or only mild LVSD, 111 (15.8%) had either moderate or severe valvopathy (dotted boxes), suggesting that in these patients, valvopathy was the primary pathology. Of those with moderate or severe LVSD, 95 (13.5%) had either no or only mild valvopathy (light grey boxes), suggesting that LVSD was the primary pathology in these patients. In 70 patients (10.5%), moderate and severe LVSD coincided with moderate or severe valvopathy (dark grey boxes), so a primary pathology may not be absolutely defined in these patients.
Table 2 illustrates that nearly a third (214, 30.4%) of all patients had no LVSD and no valvopathy. Of the patients with normal left ventricular function or only mild LVSD, 111 (15.8%) had either moderate or severe valvopathy (dotted boxes), suggesting that in these patients, valvopathy was the primary pathology. Of those with moderate or severe LVSD, 95 (13.5%) had either no or only mild valvopathy (light grey boxes), suggesting that LVSD was the primary pathology in these patients. In 70 patients (10.5%), moderate and severe LVSD coincided with moderate or severe valvopathy (dark grey boxes), so a primary pathology may not be absolutely defined in these patients.
Discussion
This paper reports an audit of a service aimed at delivering the NSF for Coronary Heart Disease. TTE-based evaluation of left ventricular function remains the gold-standard imaging modality for assessment in cases of suspected HF. It is shown to have a very high yield in both primary and secondary care.
As shown in this study and previously identified in the NSF 2000, the majority of requests for diagnostic TTE arise from primary care, which may justify the need for GPwSI-led services in primary care. The primary care service is in our opinion essential as it not only reduces the burden of diagnostic service provision on secondary care, but also identifies a significant level of valvopathy and other causes of HF, similar to the secondary care service. In addition to provision of diagnostic services, the primary care service is also equally effective at providing tailored management strategies for patients with HF.
The majority of out-patients had normal systolic function. GPs are more likely to refer patients with more severe HF to a secondary care clinic than to a primary care clinic, as evidenced by the higher levels of moderate and severe LVSD in out-patients referred to the secondary care clinic.
In-patient referrals accounted for a third of TTE services. TTE on CCU is important since it not only guides optimal therapy but guides the delivery of evidence-based medicine (most patients in post-myocardial infarction HF studies underwent assessment of LVSD early post-myocardial infarction, e.g. Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study [EPHESUS]17). Ischaemic heart disease confers a four-fold increase in the risk for developing HF18 and is the underlying aetiology in about two-thirds of patients with HF caused by LVSD. Early TTE is also invaluable on CCU for the diagnosis of a small but significant number of patients with LVTh.
Comprehensive initial evaluation by a clinician is useful since many patients with diagnosed HF can be successfully followed up in a nurse-led clinic for review, drug initiation or drug titration (either in primary or secondary care), with ad hoc input from a clinician (since both clinics run in parallel).
Non-CCU in-patients had the highest proportion of moderate and severe LVSD. Possible explanations for this are that these patients are more likely to have co-morbidities e.g. renal failure (thus increasing the likelihood of co-existent LVSD); and secondly this group is likely to contain patients with more severe HF, a characteristic of which is recurrent hospitalisation.
Unlike LVSD, out-patients had similar levels of valvopathy whether referred to primary or secondary care clinics. CCU patients were strikingly similar to out-patients in distribution of valvopathy severity, reflecting that these patients are after all drawn directly from the community, selected for LVSD rather than valvopathy. As with LVSD, the most fertile group of patients in whom valvopathy was identified was the non-CCU in-patient group, where two-thirds had significant valvopathy. As with LVSD, this might be explained by the association of valvopathy with other co-morbidities e.g. tricuspid regurgitation in pulmonary hypertension, and hospitalisation associated with certain valvopathies per se, e.g. symptomatic aortic stenosis, precipitating syncope.
Not unexpectedly, we found a significant incidence of LVTh. Early diagnosis guided anticoagulation, which potentially reduces morbidity and mortality from thromboembolic disease. LVH was also not uncommon and reflects HF with a hypertensive aetiology. As expected in such a large TTE series, we also identified less common disorders such as atrial myxoma.
Some of our findings are unique when compared with previous work on diagnostic services for HF. We show for the first time the relationship between LVSD and valvopathy in terms of severity (table 2). The majority (60.7%) had either absent or mild valvopathy with co-existent normal systolic function or only mildly impaired LVSD. A total of 15.8% of patients had moderate or severe valvopathy with co-existent normal systolic function or mild LVSD, suggesting that in these patients, valvopathy was the primary pathology. In 13.5% of all patients either moderate or severe LVSD was coupled with either absent or mild valvopathy, suggesting that LVSD was the primary pathology in these patients. Another 10.5% of patients had moderate or severe LVSD with co-existent moderate or severe valvopathy, making the primary pathology difficult to identify in this group. In many patients, LVSD and valvular function are clearly inextricably linked, e.g. functional mitral regurgitation in LVSD or LVSD secondary to aortic stenosis.
We accept that our audit did not look for LVDD. Tissue Doppler echocardiography, which is not widely available, is probably the most sensitive imaging modality available to diagnose LVDD. Many patients with suspected HF but no evidence of LVSD are often quoted as having ‘HF-preserved ejection fraction’. After excluding many differential diagnoses, e.g. obesity, constrictive pericarditis, etc., a small proportion of the patients have true LVDD as a cause for their HF. Those opposing routine evaluation for LVDD would argue that not only is there no widely accepted definition of LVDD but there is a paucity of validated therapies for patients with LVDD. Proponents of LVDD assessment will highlight the advent of studies for patients with LVDD19 and the need to avoid misdiagnosis of patients as not having HF.
The findings of TTE in both in-patients and out-patients with suspected HF justifies the recommendation for TTE in the NSF for Coronary Heart Disease for patients with suspected HF and the development of well-structured multi-disciplinary HF services. Cardiologists responsible for such services will also be able to identify patients suitable for advanced HF therapies, e.g. device therapy. Early delivery of all HF therapies is of the utmost importance to patients with HF.
One important issue to acknowledge is that HF is not a diagnosis but a syndrome. One must accept that simply finding LVSD is not sufficient in managing patients with HF, since an aetiology must be sought once HF has been diagnosed. In some cases, as we have shown, TTE identifies an aetiology in terms of valvopathy, but in the majority of cases an aetiology must still be sought so that potentially treatable and reversible cases of HF are not overlooked.
We acknowledge that this is a retrospective analysis of a service with no data on patient morbidity and mortality, but our intention was not to assess this, rather to assess the findings in different patient groups exposed to NSF-guided HF assessment for suspected LVSD.
Conflict of interest
None declared.
Key messages
- Echocardiography reveals a significant prevalence of valvular disease in patients presenting with symptoms of heart failure
- The burden of heart failure justifies the presence of diagnostic echocardiography services in both primary and secondary care
- There are significant levels of both left ventricular systolic dysfunction and valvopathy in out-patients and in-patients with symptoms of heart failure, justifying the need for diagnostic services to treat each of these populations with equity of access
- Heart failure is a syndrome and not a diagnosis. Echocardiography may reveal valvopathy as an aetiology in some cases, but an aetiology must still be sought, even after a diagnosis of left ventricular systolic dysfunction has been made
References
- Sans S, Kestesloot H, Kromhout D. The burden of cardiovascular diseases mortality in Europe. Task force of the European Society of Cardiology on cardiovascular mortality and morbidity statistics in Europe. Eur Heart J 1997;18:1231–48.
- Davies M, Hobbs F, Davis R et al. Prevalence of left-ventricular systolic dysfunction and HF in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001;358:439–44.
- Davis RC, Hobbs FD, Kenkre JE et al. Prevalence of left ventricular systolic dysfunction and HF in high risk patients: community based epidemiological study. BMJ 2002;16:1156.
- Cowie MR, Mosterd A, Wood DA et al. The epidemiology of HF. Eur Heart J 1997;18:208–25.
- Mosterd A, Hoes AW, de Bruyne MC et al. Prevalence of HF and left ventricular dysfunction in the general population. The Rotterdam Study. Eur Heart J 1999;20:447–55.
- Cowie MR, Wood DA, Coats AJW et al. Incidence and aetiology of HF: a population-based study. Eur Heart J 1999;20:421–8.
- McMurray JJV, Hart W, Rhodes G. An evaluation of the cost of HF to the NHS in the UK. Br J Med Econ 1993;6:99–110.
- Wheeldon NM, MacDonald TM, Flucker CJ, McKendrick AD, McDevitt DG, Struthers AD. Echocardiography in chronic HF in the community. Q J Med 1993;86:17–23.
- Bristow MR, Gilbert EM, Abraham WT et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic HF (MOCHA investigators). Circulation 1996;94:2807–16.
- Cowie MR, Wood DA, Coats AJ et al. Survival of patients with a new diagnosis of HF: a population based study. Heart 2000;83:505–10.
- Hobbs FD, Jones MI, Allan TF, Wilson S, Tobias R. European survey of primary care physician perceptions on HF diagnosis and management (Euro-HF). Eur Heart J 2000;21:1877–87.
- Hickling JA, Nazareth I, Rogers. The barriers to effective management of HF in general practice. Eur Heart J 2000;21:1877–87. Br J Gen Pract 2001;51:615–18.
- McClure S, Caruana L, Davie AP, Goldthorp S, McMurray JJ. Cohort study of plasma natriuretic peptides for identifying left ventricular systolic dysfunction in primary care. BMJ 1998;317:516–19.
- Francis CM, Caruana L, Kearney P et al. Open access echocardiography in management of HF in the community. BMJ 1995;310:634–6.
- Department of Health. National Service Framework for Coronary Heart Disease, Chapter 6. London: HMSO, 2000.
- Royal College of Physicians. Chronic Heart Failure. National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians, 2003.
- Aggarwal A, Coca SG, Buller GK, Blaustein DA, Schwenk MH, Pitt B; the EPHESUS Investigators. Eplerenone in Patients with Left Ventricular Dysfunction. N Engl J Med 2003;349:88–9.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive HF: the Framingham study. N Engl J Med 1971;285:1441–6.
- Yusuf S, Pfeffer MA, Swedberg K et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic HF and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777–81.
