Professor John Camm addressed the role of the late sodium current as a new target in ischaemic heart disease (IHD). The sodium channel itself spans the cardiac myocyte membrane and allows sodium transport from outside the cell to the inside of the cell.
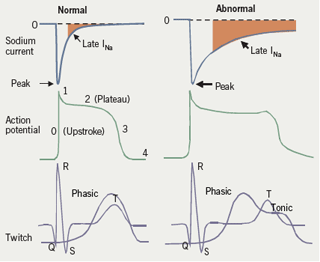
The sodium current peaks at the onset of the action potential and continues throughout systole, with a so-called late component, or late INa (see figure 1), which decays gradually. It is responsible in part for maintaining the plateau of the action potential, and it is therefore one of the determinants of the QTc interval.
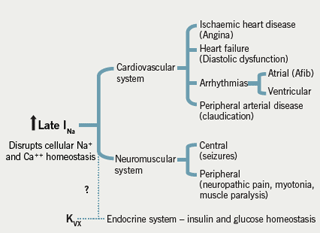
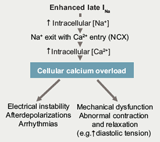
“This particular channel may be responsible for a number of pathologies and may be a target in a variety of diseases” in addition to angina, said Professor Camm. He explained a pathological paradigm of the inability of the sodium channel to inactivate, which occurs in both acquired and congenital conditions. Inability of sodium channel inactivation, ie ‘enhanced late INa’ (figure 2), leads to greater influx of sodium into the cell and an increased level of intracellular sodium. This in turn engages the sodium/calcium exchange mechanism: sodium leaves the cell and calcium enters, giving rise to calcium overload. This defective sodium channel gating therefore leads to calcium overload and a wide array of ‘channelopathy’ disorders, including ischaemia and heart failure and the congenital Long QTc 3 syndrome (LQT3) which is associated with a specific genetic abnormality. Similar abnormalities may occur in other muscles and nerves, giving rise to seizures and myotonia and neuropathic pain (figure 3).
What is ranolazine?
Professor Camm went on to describe how the novel antianginal anti-ischaemic agent, ranolazine is thought to selectively inhibit the late INa and to attenuate the abnormalities of ventricular repolarisation and electrical instability associated with ischaemia and heart failure. This inhibition is concentration-dependent. Ranolazine has been shown to cause modest QTc interval prolongation, but without causing torsade de pointes. It does not slow heart rate or decrease blood pressure to a clinically relevant degree. Additionally, it does not decrease left ventricular (LV) systolic pressure or LV dp/dt (a measure of ventricular function) or increase coronary blood flow.
Studies in which increasing doses of ranolazine were administered to volunteers confirm that the drug does not have chronotropic effects. This is confirmed in studies where ranolazine (compared to placebo) produced no increases in rate-pressure product (RPP) during exercise; in contrast, beta blockade decreases RPP.

Professor Camm then reviewed clinical studies with ranolazine. This agent has been evaluated in three principal efficacy trials (table 1).
MARISA compared three doses of ranolazine– 500 mg, 1,000 mg and 1,500 mg twice daily– with placebo in 191 randomised patients. A clear dose-response relationship was seen in standard exercise testing parameters i.e. exercise duration, time to 1 mm ST-segment depression, and time to angina onset, assessed at trough and at peak. Improvements with ranolazine were significant versus placebo at both peak and trough (the latter being the important criterion for regulatory purposes). There was only a minor change in RPP whereas “the ST-segment change was significant and highly dose-related at each point during the exercise test,” said Professor Camm.
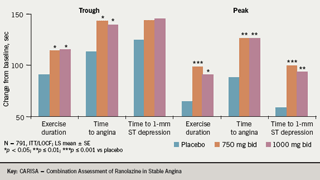
CARISA randomised patients to ranolazine 750 mg and 1,000 mg twice daily and placebo, on a background of atenolol 50 mg OD (43%), amlodipine 5 mg OD (31%) and diltiazem 180 mg OD (26%), respectively. This study also showed significant increases in exercise times (as well as decreased angina attacks and nitroglycerin [GTN] consumption) but “there was no dose-related change in these exercise parameters,” said Professor Camm (figure 4).
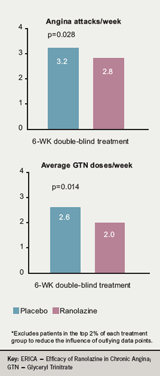
ERICA randomised patients to receive placebo or ranolazine 500 mg twice daily for one week initially before the dose was titrated up to ranolazine 1,000 mg twice daily and continued for another six weeks. All patients received amlodipine 10 mg OD with (45% of patents at baseline) or without long-acting nitrates. Frequency of angina attacks, the primary end point, and mean weekly GTN consumption were reduced significantly with ranolazine versus placebo (figure 5).
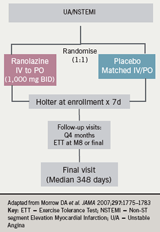
Professor Camm went on to discuss two reports from the large mortality, outcome trial, MERLIN-TIMI 36 (table 2). This was a double-blind, randomised, placebo-controlled trial conducted in 17 countries, which enrolled 6,560 patients with non-ST-elevation acute coronary syndromes within 48 hours of ischaemic symptoms.


Patients were treated with ranolazine (initially intravenously and followed by oral ranolazine 1,000 mg twice daily or matching placebo, and followed up for a median of 348 days. Follow-up assessments were conducted every four months (figure 6). The primary end point was a composite of cardiovascular death, MI or recurrent ischaemia at 12 months. Continuous ECG Holter recording was performed for the first seven days after randomisation, and a pre-specified set of arrhythmias was evaluated. The major safety end points were death from any cause and symptomatic documented arrhythmia.
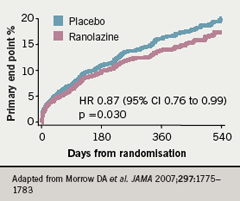
The Kaplan-Meier estimated rates of the primary end point (cardiovascular death, MI or recurrent ischaemia at 12 months) are shown in figure 7 and no significant difference was observed between ranolazine and placebo “but recurrent ischaemia was significantly reduced in the patients who were taking ranolazine,” said Professor Camm (figure 8).
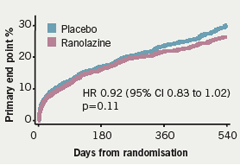
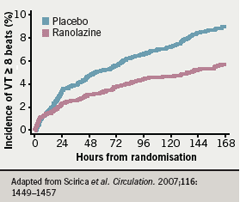
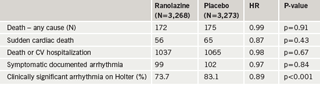
Similarly, the number of patients with worsening angina was significantly lower with ranolazine compared to placebo and there was less requirement for increased anti-anginal treatment with ranolazine (figure 9). In terms of major safety end points, “none of them is greater in association with ranolazine than with placebo,” said Professor Camm, who noted that clinically significant arrhythmias on Holter were observed in 74% of patients treated with ranolazine and in 83% of patients in the placebo group (p<0.001) (table 3). This difference was particularly noteworthy with ventricular tachycardia lasting eight beats or more; there was a “substantial reduction associated with ranolazine” (5.3% vs 8.3%;p<0.001) over seven days of Holter monitoring (figure 10).
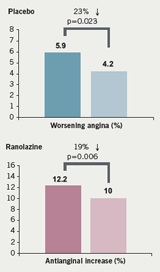
Summarising, Professor Camm said that ranolazine is thought to be a late sodium current inhibitor, which thereby reduces myocardial oxygen consumption (MVO2). It does not reduce the rate pressure product significantly but does reduce myocardial ischaemia. It has been assessed in a number of clinical trials where it shows an improvement in exercise performance, a decrease in angina attacks, GTN consumption, and a 23% risk reduction for worsening angina or ischaemia. “Ranolazine seems to be an effective and safe antianginal agent,” he concluded.
JC is an advisor to CV Therapeutics and Servier.
