The pathological paradigm: The use of a slow sodium inhibitor may improve angina pectoris. This is based on the following pathological paradigm. The transient, or peak, sodium current occurs at the beginning of the action potential: the rapid influx of sodium causes the upstroke of the action potential. This current does not immediately die away: it has a tail, known as the late, delayed or slow sodium current. In an abnormal situation, the late sodium current can be enhanced. Normally this current transports about half the sodium from outside the cell to the inside but under certain situations, when it becomes enhanced, it transports far more sodium into the cell and sodium accumulates within the cell. An enhanced current prolongs the action potential and, with it, the QT interval. There is a mechanical consequence to this enhancement: instead of the simple phasic twitch when myocardium is activated, the pattern of mechanical contraction is phasic followed by a tonic component. This tonic component extends into diastole, meaning that muscle is still tense during the early part of diastole.
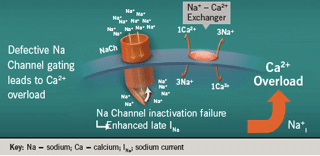
Figure 1 shows a diagram of the sodium channel. Inadequate inactivation of this channel leads to higher intracellular sodium levels. High levels of sodium then exchange with calcium, leading to intracellular calcium overload. Inactivation failure of the sodium channel occurs in several clinical situations in the heart, particularly in ischaemia and heart failure, and also in a congenital condition known as long QT3 syndrome, where the late sodium channel continues to prolong the QT interval.
Ischaemia leads to late sodium current activation, which leads to calcium overload, which, in turn, leads to increased myocardial tension and further ischaemia.
We know that angina occurs when oxygen demand outstrips the oxygen supply. Reduced oxygen supply can occur through vasospasm, thrombus and atherosclerosis, for example. This ischaemia gives rise to a sodium-induced calcium overload leading to impaired myocardial diastolic relaxation, which feeds back onto both the demand (increased left ventricular end-diastolic pressure) and supply (decreased diastolic flow) of myocardial oxygen. We can treat angina pectoris in a variety of ways, by reducing afterload (calcium channel blockers [CCBs]), reducing heart rate (beta blockers, If blocker), reducing contractility (beta blockers), reducing preload (nitrates [GTN]), or by preventing vasospasm (GTN, CCBs), thrombus (aspirin) and atherosclerosis (statins). But a new possibility now exists: use of a late sodium channel current inhibitor.
The late sodium current inhibitor, ranolazine, has hardly any effect on the peak sodium current but it can dramatically reduce the late sodium current1 in situations where there is an increase in the late sodium current, such as in failing myocytes.
Moss2 demonstrated convincingly that ranolazine affects the slow sodium current in five patients with long-QT type-3 syndrome (LQT3), where a molecular abnormality causes an increase in slow sodium current. When ranolazine was infused into these patients, the QT interval was reduced as the ranolazine concentration increased. When the ranolazine infusion was switched off, the QT interval increased again as the ranolazine concentration fell.
Results in angina
The beta blocker, atenolol, was compared with ranolazine in a study of 154 patients with chronic stable angina pectoris3. Immediate-release ranolazine 400mg tds was used in this trial. * Atenolol prolonged exercise duration, reducing the rate-pressure product. Ranolazine was equally effective, if not more effective, at increasing exercise duration but it did not affect heart rate, blood pressure or the rate-pressure product. Ranolazine was indistinguishable from atenolol for time to onset of angina and ST-segment depression.
Ranolazine also has anti-ischaemic properties. For example, in one study using SPECT MPI polar scans, the reversible perfusion defect size on exercise reduced from 25% to 10%, and in a group of 21 patients the ischaemia perfusion defect
size roughly halved after treatment with ranolazine4.
The MARISA (Monotherapy Assessment of Ranolazine In Stable Angina) study was the first dose-response study conducted with prolonged-release ranolazine5. It compared the effects of various different doses of ranolazine versus placebo on exercise duration, time to angina and time to 1mm ST-segment depression at trough and at peak levels. There was a statistically significant, dose-related increase in exercise duration.*
A second trial, CARISA (Combination Assesment of Ranolazine In Stable Angina)6, included patients with severe chronic angina who were taking standard doses of beta blocker or calcium channel blocker. They were treated for three months with 750 or 1,000 mg ranolazine twice daily compared to placebo.* Exercise testing was the main end point: statistically significant increases in exercise duration occurred in the ranolazine group but there was no dose response. Trough exercise duration increased by 115.6 seconds from baseline in both ranolazine groups versus 91.7 seconds in the placebo group (p=0.01). This study helped to derive the European maximum dosage of ranolazine of 750 mg twice daily.
This study also provided an interesting finding with regard to a marker of diabetes control in patients treated with ranolazine7. In a post-hoc analysis of the patients with diabetes included in CARISA, a reduction in glycosylated haemoglobin (HbA1c) of 0.48% was observed in patients treated with ranolazine 750 mg twice daily when adjusted against placebo (p=0.008) from baseline to week 12: the clinical relevance of this has yet to be defined.
The last of the clinical pre-registration studies for ranolazine, ERICA (Efficacy of Ranolazine in Chronic Angina)8, randomised stable patients with coronary disease and three or more angina attacks per week to ranolazine against placebo in an ascending ranolazine dose of 500 to 1,000 mg twice daily for six weeks.* Patients (n=565) were already pre-treated with amlodipine 10 mg daily. Results showed a significant reduction in the number of angina attacks per week from 3.2 with placebo to 2.8 with ranolazine (p=0.028) and reduction in average nitroglycerin doses per week from 2.6 with placebo to 2.0 with ranolazine (p=0.014).
The MERLIN-TIMI 36 trial
MERLIN-TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes) was the landmark outcome study carried out with ranolazine9 in 6,500 patients with unstable angina or non-ST elevation myocardial infarction (NSTEMI). They were randomised to receive ranolazine or placebo, initially intravenously and then orally (extended-release, 1000mg bd*), for up to one year. The primary efficacy end point for the study was cardiovascular death, myocardial infarction (MI) or recurrent ischaemia.
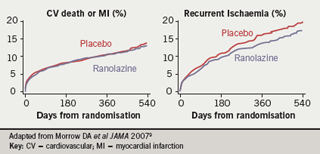
The primary end point was not met: it occurred in 21.8% of the ranolazine group and 23.5% of the placebo group (hazard ratio 0.92, p=0.11). Results for the two components of the primary end point are shown in figure 2. For death or MI, the two curves for ranolazine or placebo treatment were superimposed (hazard ratio 0.99, log-rank p=0.87). For recurrent ischaemia, there was a statistically significant reduction associated with ranolazine (16.1% for placebo versus 13.9% for ranolazine; hazard ratio 0.87; p=0.030). There was no difference between ranolazine and placebo in the risk of all-cause mortality, sudden cardiac death or frequency of symptomatic documented arrhythmias.
In a pre-specified subgroup of patients who presented with a history of chronic stable angina before their acute coronary syndrome/NSTEMI presentation, there was a highly significant reduction of recurrent ischaemia events and this also drove the reduction in the primary end point.10
These studies led to ranolazine being licensed as add-on therapy for the symptomatic treatment of patients with stable angina who are inadequately controlled or intolerant to first-line antianginal therapies.
Conflict of interest statement
JC has received speaker’s and adviser’s honoararia from Menarini, CV Therapeutics and Servier.
References
- Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog Biophy Mol Biol 2008;96:421–51.
- Moss AJ, Zareba W, Schwarz KQ et al. Ranolazine shortens repolarisation in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovas Electrophysiol 2008;19:1289–93.
- Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 2005;95:311–16.
- Venkataraman R, Belardinelli L, Blackburn B et al. Ranolazine improves myocardial perfusion in patients with coronary artery disease. ICNC9 2009 (Nuclear Cardiology and Cardiac CT), abstract 64.
- Chaitman BR, Skettino SL, Parker JO et al for the MARISA Investigators. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–82.
- Chaitman BR, Pepine CJ, Parker JO et al for the combination assessment of ranolazine in stable angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina. A randomized controlled trial. JAMA 2004;291: 309–16.
- Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27:42–8.
- Stone PH, Gratsiansky NA, Blokhin A et al for the ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine. The ERICA (Efficacy of Ranolazine in Chronic Angina) Trial. J Am Coll Cardiol 2006;48:566–75.
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes. The MERLIN-TIMI 36 randomized trial. JAMA 2007;297:1775–83.
- Wilson SR, Scirica BM, Braunwald E et al. Efficacy of ranolazine in patients with chronic angina. Observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (metabolic efficiency with ranolazine for less ischemia in non-ST-segment elevation acute coronary syndromes) 36 trial. J Am Coll Cardiol 2009;53:1510–16.
