Aldosterone/renin ratio (ARR) is commonly used to screen for primary hyperaldosteronism (Conn’s disease). A number of drugs can alter ARR measurements, thus requiring omission before testing. However, hormonal agents such as the combined oral contraceptive (COCP) or progestogen-only pill (POP) are not listed for omission. A 20-year-old woman was referred to the endocrinology team, following investigations for syncope by her cardiologist, when ARR was found to be elevated. She was taking POP (Cerelle®) while having ARR measured. After omitting POP for four weeks, plasma aldosterone concentration was reduced by 52% (from 560 pmol/L to 271 pmol/L, reference range: 100–450 pmol/L), plasma renin concentration increased by 253% (from 3.6 mU/L to 12.7 mU/L, reference range: 5.4–30 mU/L) and ARR reduced from 156 to 21 (–86.5%) (reference range: <80 suggests Conn’s unlikely). To the best of our knowledge, this is the first reported case of POP-related false-positive ARR screening for primary hyperaldosteronism. Omission of POP should, therefore, be considered in women undergoing ARR measurement.
Case presentation
A 20-year-old woman was being investigated privately for syncope in May 2017. Tilt-test showed that on standing, her heart rate increased by 30 beats/minute from baseline. She was referred to the cardiology team. Her body mass index (BMI) was 23 kg/m2 and average 24-hour ambulatory blood pressure was 141/79 mmHg. She had a normal echocardiogram and 24-hour urinary catecholamines. The patient completed the standard treadmill test. Because of hypertension detected in this patient, aldosterone/renin ratio (ARR) was performed to screen for primary hyperaldosteronism (Conn’s disease). ARR was found to be raised at 156 (reference range: <80 indicates Conn’s disease unlikely), with plasma concentration of aldosterone 560 pmol/L (reference range: 100–450 pmol/L) and plasma renin concentration 3.6 mU/L (reference range: 5.4–30 mU/L). Since Conn’s disease could not be excluded, she was referred to the endocrinology team.
On consultation, the patient reported no other medical conditions or treatment other than progestogen-only pill (POP) containing 75 µg of desogestrel (Cerelle®, Consilient Health Ltd, UK) for two years, which she was taking at the time when ARR was measured. She had normal renal, liver and thyroid functions without evidence of hypernatraemia (sodium 138 mmol/L) or hypokalaemia (potassium 4.6 mmol/L).
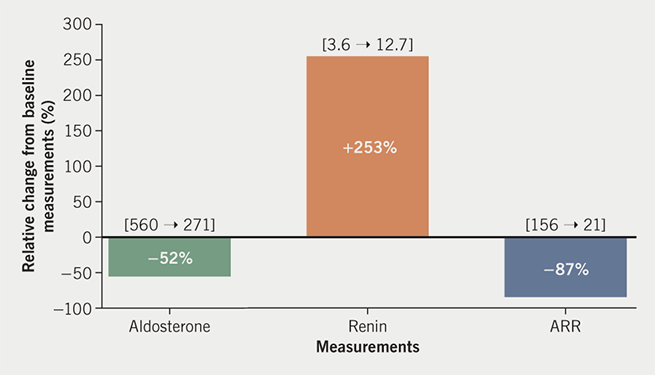
Although not routinely mentioned in published endocrine protocols,1-3 POP was suspected to potentially interfere with ARR measurement in view of the absence of other indicators to suggest primary hyperaldosteronism (suppressed renin, hypernatraemia or hypokalaemic alkalosis). She was, therefore, advised to omit POP for four weeks and to use alternative contraceptive methods to avoid unplanned pregnancy, before having ARR measurement repeated by the same immunoassays. POP-free plasma aldosterone concentration was reduced to 271 pmol/L, plasma renin concentration increased to 12.7 mU/L and ARR reduced to a normal level of 21. These changes equate to relative reduction of aldosterone from baseline level by –52%, increase in renin by +253%, and a net reduction in ARR by –87% (figure 1). Sodium (138 mmol/L) and potassium (4.6 mmol/L) levels remained exactly the same as those performed during the initial ARR screening, with normal levels of bicarbonate (27 mmol/L). During the period of investigations, the patient did not change her diet or lifestyle and was not on any medications.
Diagnosis and management
The patient was diagnosed with postural orthostatic tachycardia syndrome (PoTS) by the cardiologist. The previously raised ARR had normalised after POP omission indicating a false-positive ARR screening test associated with POP. She was reassured of the normal POP-free ARR measurement and no further endocrine investigations were necessary. The patient was advised that she could restart POP if she wished. On completion of endocrine investigations, her cardiologist prescribed midodrine at the initial dose of 2.5 mg three times daily, followed by a further increase to 5 mg three times daily for treatment of PoTS.
Discussion
The present report of a young woman shows a clear temporal association of changes in ARR with POP and its omission; ARR rose to a level where Conn’s disease could not be excluded while taking POP, followed by normalisation of ARR after omission of this contraceptive agent. To the best of our knowledge, this is the first reported case of POP-related false-positive ARR screening for primary hyperaldosteronism. We suggest that when performing ARR, omission of POP for up to four weeks should be considered to avoid false-positive results and, thus, prevent further unnecessary investigations and treatment.
Random ARR is commonly used by specialists such as endocrinologists to screen for primary hyperaldosteronism (Conn’s disease) in individuals with hypertension. A number of drugs can alter ARR measurement, thus, requiring their omission before testing (table 1).1-3 In women, the combined oral contraceptive pill (COCP) has also been shown to interfere with ARR,4 while the effect of POP on ARR is little known.
Table 1. Effects of drugs that may require omission before aldosterone/renin ratio (ARR) testing on ARR measurements
| Drug | Effects on measurements | Recommended washout period | |||
|---|---|---|---|---|---|
| Random ARR screening test | Saline infusion test | ||||
| Effects on renin concentration* | Effects on ARR | Based on Turner & Wass1 | Based on Imperial Endocrine Handbook2 | Based on both Turner & Wass1 and Imperial Endocrine Handbook2 | |
| Alpha blockers | Negligible | Negligible | Continue | Continue | Continue |
| Angiotensin-converting enzyme inhibitors | Stimulation | False negatives | 2 weeks | May stop | 2 weeks |
| Angiotensin II receptor blockers | Stimulation | False negatives | 2 weeks | May stop | 2 weeks |
| Calcium-channel antagonists | Stimulation | False negatives | 2 weeks (may continue verapamil) | May continue | 2 weeks |
| Diuretics | Stimulation | False negatives | 6 weeks | Not mentioned | 6 weeks |
| Mineralocorticoid receptor antagonists | Stimulation | False negatives | 6 weeks | 6 weeks | 6 weeks |
| Beta blockers | Suppression | False positives | 2 weeks | 2 weeks | 2 weeks |
| Clonidine | Suppression | False positives | Not specified | Not mentioned | Not specified |
| Methyldopa | Suppression | False positives | Not specified | Not mentioned | Not specified |
| Non-steroidal anti-inflammatory drugs | Suppression | False positives | Not specified | Not mentioned | Not specified |
| Combined oral contraceptive pill (oestrogen component) | Suppression† | False positives† | Not mentioned | Not mentioned | Not mentioned |
| Progestogen-only pill | Suppression‡ | False positives‡ | Not mentioned | Not mentioned | Not mentioned |
| *There is a lack of information on effects of drugs on aldosterone levels. †Based on Ahmed et al.7 ‡According to our observation: POP associates with raised aldosterone and suppression of renin resulting in elevated ARR results. Four weeks of POP omission normalised ARR measurement. |
|||||
Endogenous progesterone is an antagonist at the mineralocorticoid receptor, therefore, an increase in progesterone production, such as during the luteal phase of the menstrual cycle, leads to natriuresis and a compensatory activation of the renin–aldosterone system, and, consequently, an increase in renin and aldosterone production.4 By contrast, synthetic progestogens have variable affinity for binding to the mineralocorticoid receptor; older progestogens (e.g. medroxyprogesterone acetate and norethisterone acetate) bind with relatively low affinity, while newer ones (e.g. drospirenone) have a similar anti-mineralocorticoid effect to that of endogenous progesterone.5,6
There are a number of studies examining the effects of contraceptive hormones on renin and aldosterone. Although the effects of oestrogens are well established, it is not clear how much progestogens exert their effects on renin and aldosterone due to a number of factors, primarily because of study designs. First, studies tend to examine COCP rather than POP, therefore, the effects of oestrogen would mask those of progestogens. Second, methods of measuring renin are inconsistent – some studies use plasma renin activity while others use direct concentration; these two measures give very different ARR results in response to hormonal therapy (see below). Ahmed et al. studied 17 normotensive women treated with COCP (ethinylestradiol plus drospirenone) for three weeks and found significant increases in aldosterone and plasma renin activity, while decreases in renin concentrations were observed, leading to increases in ARR when renin concentration was used, but no change in ARR when plasma renin activity was used.7 These authors interpreted that ethinylestradiol was responsible for decreasing renin concentration while drospirenone was responsible for raising aldosterone concentration. In the same study, these authors also observed that treatment with subdermal progestin-only contraceptive implant etonogestrel (Implanon®), for either one week or six weeks, did not alter aldosterone or renin concentration in women.7
There is a lack of information on the effects of oral progestogens on direct concentration of renin or aldosterone. Pizzolo et al. reported a case of a 34-year-old woman with a false-positive ARR result associated with Yasmin (Shering S.p.A., Milan, Italy) treatment. Although Yasmin is a COCP, containing drospirenone as well as ethinylestradiol, these authors singled out the progestogen component, drospirenone, as the factor that may have interfered with ARR measurement.8 If this finding is correct then it would support our observation. We are not aware of a previous report on the effect of POP alone on ARR when direct renin concentration is measured. POP may alter aldosterone and/or renin concentration by mechanisms that are yet to be elucidated. Accuracy of immunoassay for measuring aldosterone has been questioned, but is unlikely to affect the outcome of our study since the degree of change in aldosterone (52%) between POP and POP-free period was relatively small compared with that in renin (253%); this five-fold difference raises the possibility that POP may have caused interference with direct plasma renin concentration assay, and to a lesser extent with aldosterone assay. In support of this notion, plasma renin concentration assay has been shown to suffer antibody interference, leading to a false-negative result in a 70-year-old man who was confirmed to have Conn’s disease.9
We recognise certain limitations exist in our report, since retesting ARR on restarting POP was not performed, but we feel that it was unnecessary to subject the patient to further tests which would delay and interfere with her new treatment with midodrine, and may also cause anxiety to the patient. The association of POP and ARR in a single case study, which may suffer from a random effect, should not be interpreted as causally linked. We had no control over the patient’s dietary intake but the suppressed levels of renin observed in the initial ARR screening were unlikely to be due to the patient’s sodium intake, since the accompanying aldosterone levels were elevated – studies have shown that high sodium intake leads to a reduction in both renin and aldosterone levels.10
Conclusion
In conclusion, POP appeared to cause a false-positive ARR screening test in a young woman. It may be prudent to avoid as many medications as possible while performing ARR measurements. Further investigations on a larger number of women to confirm this finding, and to establish the period of washout prior to ARR measurement, are warranted.
Key messages
- Aldosterone/renin ratio (ARR) is a standard screening test for primary hyperaldosteronism
- A number of drugs interfere with ARR measurement and, therefore, need to be omitted for testing, but the progestogen-only pill (POP) is not listed
- A young lady was found to have raised ARR while taking POP (Cerelle®), but the levels returned to normal after omission of POP for four weeks, suggesting POP is associated with false-positive ARR test
- POP may have interfered with direct renin concentration assays
- POP should be included in the list of drug omission before performing ARR to prevent further unnecessary investigations and treatment
Conflicts of interests
None declared.
Funding
None.
Patient consent
Written informed consent was obtained from the patient for publication of this case report.
References
1. Turner H, Wass J (Eds). Oxford Handbook of Endocrinology and Diabetes. Oxford: Oxford University Press, 2009. https://doi.org/10.1093/med/9780198567394.001.0001
2. Endocrine Unit Imperial College Healthcare NHS Trust Charing Cross, Hammersmith and St. Mary’s Hospitals. Endocrinology handbook. Updated: March 2016. Available from: http://gim.org.uk/Bible2016.pdf [accessed August 2019].
3. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res 2012;44:170–6. https://doi.org/10.1055/s-0031-1295460
4. Quinkler M, Meyer B, Bumke-Vogt C et al. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol 2002;146:789–99. https://doi.org/10.1530/eje.0.1460789
5. Oelkers W, Berger V, Bolik A et al. Dihydrospirorenone, a new progestogen with antimineralocorticoid activity: effects on ovulation, electrolyte excretion, and the renin-aldosterone system in normal women. J Clin Endocrinol Metab 1991;73:837–42. https://doi.org/10.1210/jcem-73-4-837
6. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endoc Rev 2012;34:171–208. https://doi.org/10.1210/er.2012-1008
7. Ahmed AH, Gordon RD, Taylor PJ, Ward G, Pimenta E, Stowasser M. Effect of contraceptives on aldosterone/renin ratio may vary according to the components of contraceptive, renin assay method, and possibly route of administration. J Clin Endocrinol Metab 2011;96:1797–804. https://doi.org/10.1210/jc.2010-2918
8. Pizzolo F, Pavan C, Corrocher R, Olivieri O. Laboratory diagnosis of primary aldosteronism, and drospirenone–ethinylestradiol therapy. Am J Hypertens 2007;20:1334–7. https://doi.org/10.1016/j.amjhyper.2007.08.009
9. Powlson AS, Oddy S, Halsall DJ, Moran C, Gurnell M. Renin assay interference may conceal the diagnosis of primary aldosteronism. Endocrine Abstracts 2017;50:CC04. https://doi.org/10.1530/endoabs.50.CC04
10. Bayard F, Cooke CR, Tiller DJ et al. The regulation of aldosterone secretion in anephric man. J Clin Invest 1971;50:1585–95. https://doi.org/10.1172/JCI106646
