Immunoglobulin G4-related disease (IgG4-RD) is a systemic fibro-inflammatory immune-mediated disease, which has been defined in the past few years. IgG4-RD affects various organs and leads to a variety of clinical manifestations. As it is a relatively newly defined entity, new manifestations are now being recognised and reported. We describe a case involving the cardiovascular system.
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is an immunologic fibro-inflammatory systemic disease that may affect different parts of the body in different ways. The involved organs or structures are infiltrated by inflammatory IgG4-positive plasma cells, which, if untreated, result in fibrosis and permanent organ damage.1
Case summary
A 54-year-old woman presented with recurrent breast cancer within 10 years of her original diagnosis. Her initial breast disease was a right-side ductal carcinoma in situ (DCIS) treated with lumpectomy, followed by radiation and tamoxifen. Her past history was significant for Graves’ disease, autoimmune hypophysitis with no biochemical evidence of hypopituitarism, asthma, chronic dacryosialadenitis, nasal polyps, glucose intolerance and dyslipidaemia. She had never been diagnosed with IgG4-RD.
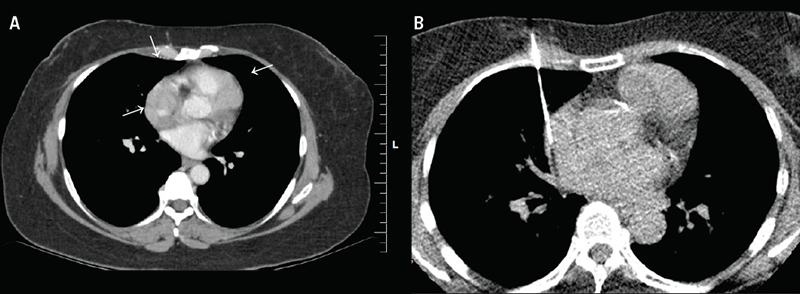
She was noted to have right recurrent breast disease and another suspicious lesion in the contralateral breast by routine surveillance. Work-up for new breast lesions and re-staging chest/abdomen/pelvic computed tomography (CT) examinations demonstrated lobulated soft tissue masses. One measuring 2.5 cm in maximum thickness encircled the lower superior vena cava (SVC), right atrial appendage, and right inferior pulmonary vein. A similar, but separate, mass measuring 2.9 cm abutted the superior surface of the right ventricle extending along the interventricular groove, involving part of the left anterior descending (LAD) coronary artery and adjacent pericardial recesses.
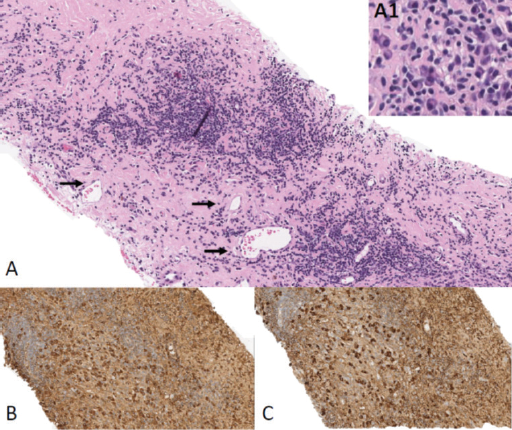
The initial working differential diagnoses were metastatic breast carcinoma and lymphoma, with cardiac sarcoma as a less likely consideration. There was no other evidence of intrathoracic metastatic disease. A CT-guided biopsy of the mass (figure 1) showed nodular lymphoplasmacytic aggregates embedded in fibrous stroma. The plasma cells were the predominant cell population and showed no evidence of monoclonality by kappa or lambda immunostains. While no obvious obliterative venulitis was observed, IgG4-positive plasma cells were clearly increased. Based on the diagnostic criteria (Boston 2012) this was highly suggestive of IgG4-related disease (figure 2).2
All lab tests, including troponin level and d-dimer, were negative. Her electrocardiogram (ECG) showed sinus rhythm with no ischaemic changes. A chest X-ray was normal.
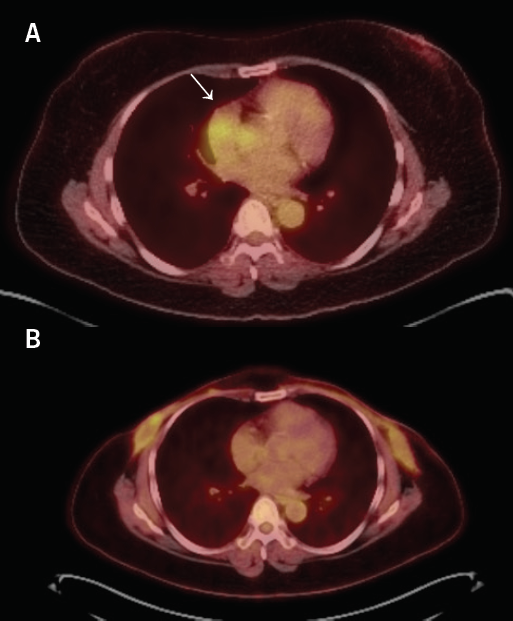
A positron-emission tomography (PET) scan was performed showing mild-to-moderate fluorodeoxyglucose (FDG) uptake in the soft tissue encasing the lower SVC, in the left mediastinum encasing the LAD and adjacent to the right inferior pulmonary vein, corresponding to the biopsy-proven IgG4 disease. At this point the serum IgG4 concentration level was (2.744 g/L; normal values 0.01–1.40 g/L).
There was a small focus of mild FDG uptake around the retroareolar clip in the right breast, which corresponded to the site of the biopsy-proven recurrent breast malignancy. She was started on prednisone and is concurrently undergoing treatment for breast cancer. She underwent right breast mastectomy because of invasive ductal carcinoma and also left breast lumpectomy and sentinel node biopsy for multi-focal DCIS. She ultimately underwent a completion left breast mastectomy. A recent follow-up PET CT scan five months after initiation of prednisone demonstrated complete metabolic response of cardiac masses, as well as size reduction (figure 3).
Discussion
IgG4-positive plasma cells infiltration and high serum IgG4 concentration were first suggested to be associated with sclerosing autoimmune pancreatitis in 2001. Later in 2003, it was proposed to be a systemic autoimmune disease. Since then, IgG4-RD has been reported involving many organs including meninges, hypophysis, brain and peripheral nerves, orbits, lacrimal and salivary glands, thyroid, bronchi, lungs, lymph nodes, mediastinum, retroperitoneum, heart and great vessels, liver and pancreas, gall bladder and bile ducts, kidneys, prostate, testis, breast, and skin.3
The IgG4-RD may affect the cardiovascular system in different ways, including involvement of coronary arteries, heart valves, myocardium, pericardium, aorta and peripheral vessels.4 IgG4-RD may induce coronary artery stenosis, either by tumour formation around coronary arteries, or by peri-arterial soft tissue thickening.5 Heart valves may be affected by infiltration of IgG4-positive plasma cells, causing valvular stenosis and regurgitation.6 Infiltration of pericardium by IgG4-positive plasma cells causes pericardial thickening.7 IgG4-RD can cause different morphologic changes in the aorta and peripheral vessels.8 The IgG4-RD may also present as cardiac masses.9 Induction therapy for IgG4-RD includes glucocorticoids, with one series documenting disease remission in 90% of cases.10 In patients with refractory disease, anti-rheumatic drugs can be considered (i.e. azathioprine, methotrexate or mycophenolate mofetil), though consensus guidelines highlight the lack of data supporting this.11 Serial measurements of IgG4 levels and imaging is helpful to ensure disease quiescence after initiation of therapy and during the maintenance period.
Conclusion
IgG4-RD is a systemic immune-mediated disease, which has been defined in the past few years and can involve every organ in the body, including the cardiovascular system. In cases of cardiac, pericardial or vascular involvement by masses or infiltrative tissues, IgG4-RD should be considered in differential diagnosis.
Key messages
- The cardiac manifestations of immunoglobulin G4-related disease (IgG4-RD) include cardiac mass, coronary artery stenosis, valvulopathy, myocardial and pericardial infiltration, aortitis, and peripheral vessel involvement
- The differential diagnosis for enhancing and fluorodeoxyglucose (FDG)-avid cardiac masses should include IgG4-RD, in addition to lymphoma, sarcoma, and metastatic disease
Conflicts of interest
None declared.
Funding
None.
Patient consent
Written consent for submission and publication of this case report including image(s) and associated text was obtained from the patient in line with COPE (Committee on Publication Ethics) guidance.
References
1. Karim F, Loeffen J, Bramer W et al. IgG4-related disease: a systematic review of this unrecognized disease in pediatrics. Pediatr Rheumatol Online J 2016;14:18. https://doi.org/10.1186/s12969-016-0079-3
2. Deshpande V, Zen Y, Chan JK et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. https://doi.org/10.1038/modpathol.2012.72
3. Dy RV, Atlas SA. So many organs, 1 diagnosis: IgG4-related disease. Am J Med 2014;127:195–7. https://doi.org/10.1016/j.amjmed.2013.12.008
4. Mavrogeni S, Markousis-Mavrogenis G, Kolovou G. IgG4-related cardiovascular disease. The emerging role of cardiovascular imaging. Eur J Radiol 2017;86:169–75. https://doi.org/10.1016/j.ejrad.2016.11.012
5. Otani T, Matsumoto Y, Inoue K, Tanaka H, Hamada M. Morphological characterization of coronary arteries in immunoglobulin G4-related disease estimated by using computed tomography and magnetic resonance imaging. Int J Cardiol 2016;215:111–13. https://doi.org/10.1016/j.ijcard.2016.04.060
6. Besik J, Pirk J, Netuka I et al. Aortic and mitral valve replacement due to extensive inflammatory immunoglobulin G4-related pseudotumor. Ann Thorac Surg 2015;100:1439–41. https://doi.org/10.1016/j.athoracsur.2014.12.051
7. Mori K, Yamada K, Konno T et al. Pericardial involvement in IgG4-related disease. Intern Med 2015;54:1231–5. https://doi.org/10.2169/internalmedicine.54.3856
8. Alba MA, Milisenda J, Fernandez S et al. Small-vessel vasculitis with prominent IgG4 positive plasma cell infiltrates as potential part of the spectrum of IgG4-related disease: a case report. Clin Exp Rheumatol 2015;33(suppl 89):S138–S141. Available from: https://www.clinexprheumatol.org/abstract.asp?a=8378
9. Kusunose K, Hotchi J, Takagawa Y et al. Serial imaging changes during treatment of immunoglobulin G4-related disease with multiple pseudotumors. Circulation 2015;131:1882–3. https://doi.org/10.1161/CIRCULATIONAHA.115.015638
10. Ebbo M, Daniel L, Pavic M et al. IgG4-related systemic disease: features and treatment response in a French cohort: results of a multicenter registry. Medicine (Baltimore) 2012;91:49–56. https://doi.org/10.1097/MD.0b013e3182433d77
11. Khosroshahi A, Wallace ZS, Crowe JL et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688–99. https://doi.org/10.1002/art.39132
