At the Association of Cardiovascular Nursing & Allied Professions conference in June, Professor Tiny Jaarsma called for a pillar of self-care to be added to heart failure (HF) care guidelines.1 Taylor et al.2 agree that HF care needs an additional pillar and suggest cardiac rehabilitation. Currently, the HF pillars are focused on medications shown to improve the outcomes of people with heart failure with reduced ejection fraction (HFrEF),3 but other key non-pharmacological interventions are not considered. Also, the pillars of care do not provide guidance for people with heart failure with preserved ejection fraction (HFpEF), which represent 50% of the HF population.4 While these pillars focus on a single important aspect of care, they do not capture the totality of HF management or illness types. A focus on interventions to improve HF management is welcome but can obscure the challenges that treatment – as well as illness – impose on patients and their carers.

Burden of treatment is a concept that describes the balance between an individual’s capacity (their abilities and available resources) and the workload (tasks assigned by healthcare professionals) related to treating their illness.5 When workload exceeds capacity, engagement with self-care, quality of life and clinical outcomes worsen.6–9
Research in people with HF has shown a relationship between symptom severity and the difficulty reported with workload associated with their illness (treatment burden).10,11 Medications were reported as part of ‘troublesome self-care’.12 But it appears that the burden lies not in the act of taking the medications. In both Norway and the UK, those with HF rated the treatment burden associated with taking medications as very easy.10,11 The parallel qualitative research highlighted that those respondents reported treatment burden as influenced by factors like carers doing the work of medication management, or the high value assigned to that work. These helpful factors were not captured in the questionnaire used to measure treatment burden.13 In interviews, people with HF reported the high treatment burden involved in required interactions with inaccessible healthcare professionals, medication side effects, or in what taking those medications means.12,13
“I remember saying to a doctor do I have to keep taking these tablets? I’m fed up you know?” (B001 male 52 years)13
Self-care
Self-care is recommended in the guidelines as part of the management for HF,4 and has been shown to improve patient outcomes. However, clinicians often don’t consider the work required to manage complex treatment regimens, especially for older patients with multiple comorbidities. Those with HF are believed by clinicians to have poor self-care practices.14 This may relate to a lack of support and information provided by healthcare professionals (HCPs). In the UK, in a sample of all types of HF, around 50% of participants reported not being told about core self-care elements (diet or exercise) by any HCP. In those that had been advised to change their diet or begin exercise, these activities were also reported to cause greater treatment burden.10
In both the UK and Norway, people with HF reported higher treatment burden around activities related to interaction with healthcare services, e.g. accessing specialists, getting appointments, and primary-care follow-up.10,11
“I can’t remember ever seeing the same doctor twice. I wish I had, that would have felt much safer.” (P.15, NYHA II)12
Care pathways

Care pathways in healthcare systems,15 and the nature of clinical interactions within that system,7,12,13,16,17 can influence treatment burden. Insufficient, complex, and inaccessible healthcare services add significantly to patients’ treatment burden, likely contributing to poor outcomes.
The national heart failure audit in the UK,18 highlighted the deficiencies of current care pathways. Of patients with HFrEF admitted to hospital for HF exacerbation, only 56% are discharged on core HF medications, 32% receive cardiology follow-up, and 10% are referred to cardiac rehabilitation.18
The lack of established care pathways for people with HFpEF means half of the population have no routine access to specialist care.19,20 The lack of care pathways leads to misconceptions in both clinicians and patients. Clinicians have reported a lack of knowledge around the diagnosis, management, and illness perceptions related to HF.19,21,22 Patients have reported that they believe that their HF isn’t serious, that nothing can be done, or other things are more important.21
“I nursed my husband through Alzheimer’s […] So I just said to the doctor, forget it [pursuing HFpEF diagnosis], put it on hold.” (P006, female, 82 years)21
Shifting the pillars
There is a call for more HF specialists to meet the growing prevalence of HF,23 an acknowledgement that new ways of working are required,24 and that changes to HF care pathways are needed.25–27 The prevalence and demands of HF, including HFpEF, are expected to rise.18,28
The British Society of Heart Failure has announced a campaign ‘25in25’ to reduce the mortality of HF through prevention, early diagnosis, specialist care and patient empowerment.29 But to ensure any changes made by this campaign do not create further health inequalities or overwhelming burden on either patients or clinicians, the pillars of HF care require a shift from the current singular focus.
The focus of the current pillars of HF treatment is on the medical management of those with HFrEF, ignoring 50% of people with HF, self-care regimens, and deficits in the current care pathways. To reduce burden of treatment for people with HF a more inclusive framework for the management of those with HF is needed (figure 1). A foundation of sufficient numbers of knowledgeable HCPs is paramount to improving HF management. Healthcare providers should implement pillars of care for all HF types, but those pillars should include more than just medication optimisation.1,2,6 A broader view of HF care, which is more inclusive and considers the implicit burden created by HF and its treatment for both patients and clinicians, may be the key to future HF care (table 1).
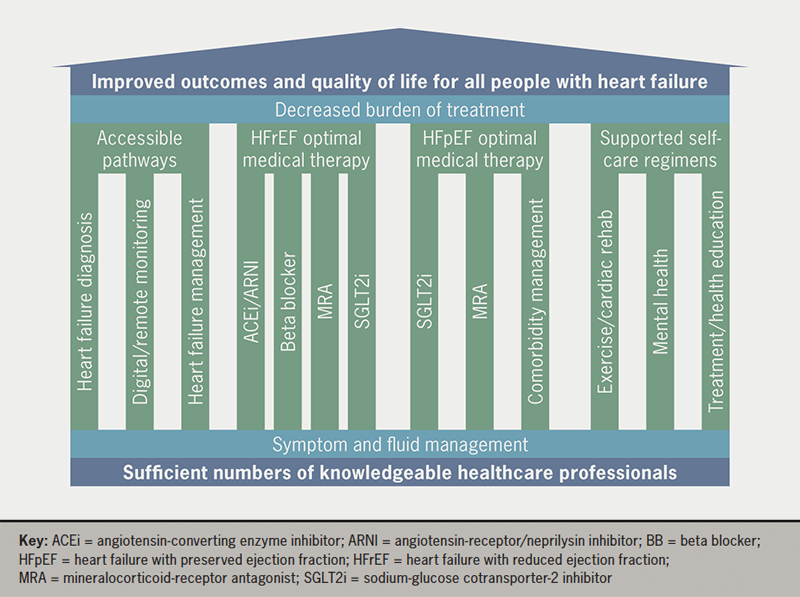
Table 1. Description of pillars of care for heart failure
| Pillars of care | Burdens alleviated |
|---|---|
| Accessible pathways for diagnosis and management for all HF types |
|
| Optimal medical therapy for HFrEF (ACEi/ARNI, BB, MRA, SGLT2i) | |
| Optimal medical therapy for HFpEF (SGLT2i, MRA, comorbidity management) |
|
| Supported self-care regimens including cardiac rehabilitation | |
| Key: ACEi = angiotensin-converting enzyme inhibitor; ARNI = angiotensin-receptor/neprilysin inhibitor; BB = beta blocker; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid-receptor antagonist; SGLT2i = sodium-glucose cotransporter-2 inhibitor | |
These pillars will hold up the goal of improved outcomes and better quality of life for people with HF in a more patient-centred approach, while simultaneously reducing the treatment burden.
Conflicts of interest
None.
Funding
This work was supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration (ARC) Wessex. The views expressed are those of the author and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Acknowledgements
Thank you to Professors Carl May and Christi Deaton for their comments on drafts of this editorial.
References
1. Jaarsma T. Self-care of heart failure patients: practical management recommendations. ACNAP Congress 2023, Edinburgh, 23 June 2023.
2. Taylor RS, Dalal HM, Zwisler AD. Cardiac rehabilitation for heart failure: ‘Cinderella’ or evidence-based pillar of care? Eur Heart J 2023;44:1511–18. https://doi.org/10.1093/eurheartj/ehad118
3. Sam S, Melanie M, Klaus KW. Four pillars of heart failure: contemporary pharmacological therapy for heart failure with reduced ejection fraction. Open Heart 2021;8:e001585. https://doi.org/10.1136/openhrt-2021-001585
4. McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
5. May CR, Eton DT, Boehmer K et al. Rethinking the patient: using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv Res 2014;14:281. https://doi.org/10.1186/1472-6963-14-281
6. May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339:b2803. https://doi.org/10.1136/bmj.b2803
7. Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med 2011;9:235–43. https://doi.org/10.1370/afm.1249
8. Austin RC, Schoonhoven L, Clancy M, Richardson A, Kalra PR, May CR. Do chronic heart failure symptoms interact with burden of treatment? Qualitative literature systematic review. BMJ Open 2021;11:e047060. https://doi.org/10.1136/bmjopen-2020-047060
9. May CR, Cummings A, Myall M et al. Experiences of long-term life-limiting conditions among patients and carers: what can we learn from a meta-review of systematic reviews of qualitative studies of chronic heart failure, chronic obstructive pulmonary disease and chronic kidney disease? BMJ Open 2016;6:e011694. https://doi.org/10.1136/bmjopen-2016-011694
10. Austin RC, Schoonhoven L, Koutra V, Richardson A, Kalra PR, May CR. SYMptoms in chronic heart failure imPACT on burden of treatment (SYMPACT): a cross-sectional survey. ESC Heart Fail 2022;9:2279–90. https://doi.org/10.1002/ehf2.13904
11. Nordfonn OK, Morken IM, Bru LE, Larsen AI, Husebo AML. Burden of treatment in patients with chronic heart failure – a cross-sectional study. Heart Lung 2021;50:369–74. https://doi.org/10.1016/j.hrtlng.2021.02.003
12. Nordfonn OK, Morken IM, Bru LE, Husebo AML. Patients’ experience with heart failure treatment and self-care – a qualitative study exploring the burden of treatment. J Clin Nurs 2019;28:1782–93. https://doi.org/10.1111/jocn.14799
13. Austin RC, Schoonhoven L, Richardson A, Kalra PR, May CR. Qualitative interviews results from heart failure survey respondents on the interaction between symptoms and burden of self-care work. J Clin Nurs 2023;32:4649–62. https://doi.org/10.1111/jocn.16484
14. Riegel B, Lee CS, Dickson VV. Self care in patients with chronic heart failure. Nat Rev Cardiol 2011;8:644–54. https://doi.org/10.1038/nrcardio.2011.95
15. Lippiett KA, Richardson A, Myall M, Cummings A, May CR. Patients and informal caregivers’ experiences of burden of treatment in lung cancer and chronic obstructive pulmonary disease (COPD): a systematic review and synthesis of qualitative research. BMJ Open 2019;9:e020515. https://doi.org/10.1136/bmjopen-2017-020515
16. Jani B, Blane D, Browne S et al. Identifying treatment burden as an important concept for end of life care in those with advanced heart failure. Curr Opin Support Palliat Care 2013;7:3–7. https://doi.org/10.1097/SPC.0b013e32835c071f
17. Cardona M, Sav A, Michaleff ZA, Thomas ST, Dobler CC. Alignment of doctors’ understanding of treatment burden priorities and chronic heart failure patients’ experiences: a nominal group technique consultation. Patient Prefer Adherence 2023;17:153–65. https://doi.org/10.2147/PPA.S385911
18. National Institute for Cardiovascular Outcomes Research. National heart failure audit. Leicester: NICOR, 2023. Available from: https://www.nicor.org.uk/heart-failure-heart-failure-audit/
19. Sowden E, Hossain M, Chew-Graham C et al. Understanding the management of heart failure with preserved ejection fraction: a qualitative multiperspective study. Br J Gen Pract 2020;70:e880–e889. https://doi.org/10.3399/bjgp20X713477
20. Masters H, Freeman J, Dixon S. Variable structure and provision of guideline-based care in specialist heart failure centres in the UK: a survey of 100 health professionals. Br J Card Nurs 2020;15:1–11. https://doi.org/10.12968/bjca.2020.0019
21. Forsyth F, Blakeman T, Burt J et al. Cumulative complexity: a qualitative analysis of patients’ experiences of living with heart failure with preserved ejection fraction. Eur J Cardiovasc Nurs 2023;22:529–36. https://doi.org/10.1093/eurjcn/zvac081
22. Hossain MZ, Chew-Graham CA, Sowden E et al. Challenges in the management of people with heart failure with preserved ejection fraction (HFpEF) in primary care: a qualitative study of general practitioner perspectives. Chronic Illn 2022;18:410–25. https://doi.org/10.1177/1742395320983871
23. Masters J, Barton C, Blue L, Welstand J. Increasing the heart failure nursing workforce: recommendations by the British Society for Heart Failure Nurse Forum. Br J Card Nurs 2019;14:1–12. https://doi.org/10.12968/bjca.2019.0109
24. Morton G, Masters J, Cowburn PJ. Multidisciplinary team approach to heart failure management. Heart 2018;104:1376. https://doi.org/10.1136/heartjnl-2016-310598
25. Desai AS, Stevenson LW. There must be a better way: piloting alternate routes around heart failure hospitalizations. J Am Coll Cardiol 2013;61:127–30. https://doi.org/10.1016/j.jacc.2012.10.015
26. Wierda E, van Veghel D, Hirsch A, de Mol B. Heart teams in the Netherlands: from teamwork to data‑driven decision-making. Neth Heart J 2020;28(suppl 1):73–7. https://doi.org/10.1007/s12471-020-01452-8
27. Wand AL, Russell SD, Gilotra NA. Ambulatory management of worsening heart failure: current strategies and future directions. Heart Int 2021;15:49–53. https://doi.org/10.17925/HI.2021.15.1.49
28. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342–56. https://doi.org/10.1002/ejhf.1858
29. Lawson C, Barton C. Changing the trajectory of heart failure and saving lives: a look at the 25in25 global initiative. Br J Card Nurs 2023;18:1–2. https://doi.org/10.12968/bjca.2023.0034
