Angiotensin II receptor blockers (ARBs) are a class of pharmaceutical agents that modulate the renin-angiotensin-aldosterone system (RAAS), which is responsible for blood pressure (BP) regulation and fluid and electrolyte homeostasis.
Differentiation in pharmacokinetics and pharmacodynamics
Losartan was the first ARB to be approved for clinical use. There are currently seven ARBs on the market: losartan, valsartan, candesartan, irbesartan, olmesartan, eprosartan and telmisartan. Despite belonging to the same drug class, these ARBs vary in some aspects of their chemical structure. This leads to important differences in pharmacokinetic and pharmacodynamic characteristics, which may be observed in terms of active metabolite, bioavailability, volume of distribution, terminal half-life, hepatic/renal elimination and protein binding (table 1). For example, losartan and candesartan are both pro-drugs, but losartan requires cytochrome P450-mediated biotransformation to yield the active moiety EXP-3174, while candesartan cilexetil is rapidly converted to candesartan by ester hydrolysis during absorption from the gastrointestinal tract.
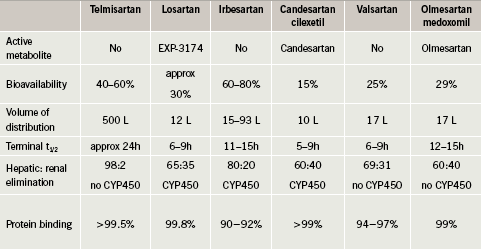
The mechanisms of antagonism also vary between the ARBs: some show insurmountable antagonism (e.g. candesartan) and others show surmountable antagonism (e.g. losartan). Insurmountable antagonism is characterised by long-lasting inhibition, slow dissociation, irreversible binding, conformational changes and potentially internalisation, whereas
surmountable antagonism is characterised by short-lasting inhibition and fast, reversible binding.1 Losartan only binds at two angiotensin II type 1 receptor (AT1) sites, compared to candesartan which shows high binding affinity at four sites.2 It is suggested that the tight binding and slow dissociation of candesartan from the AT1 receptor may account for the magnitude of the antihypertensive efficacy of candesartan and for its long duration of action, inducing long-lasting suppression of the RAAS3, 4(figure 1).
Differences in ARB efficacy
It has long been established that individuals with elevated BP are at greater risk for cardiovascular or cerebrovascular events, such as stroke, myocardial infarction and heart failure. Epidemiological evidence and findings from outcome trials indicate that the relative risk of cardiovascular events is related to BP levels in a continuous and linear manner.5 A meta-analysis of 61 cohort studies, together with the results of a meta-analysis of 147 randomised trials,6,7 suggested that when a single BP-lowering drug at a standard dose reduces diastolic BP by approximately 5 mmHg, there is a resulting reduction of approximately 25% in the relative risk of coronary heart disease events and 35% in the relative risk of stroke. As such, it is logical, when initiating antihypertensive treatment, to use within any given drug class the agents most likely to produce the greatest BP reduction.
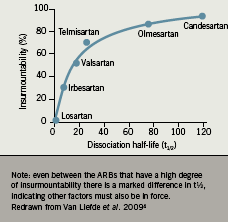
There is evidence from clinical trials that the ARBs differ in their duration of action. When the mean change from baseline in systolic BP was evaluated over a 36-hour period for losartan and candesartan, the BP response was seen to be stable over this period in the candesartan-treated patients, whereas a drop in efficacy was seen after 24 hours in the losartan group (figure 2).5 This finding could be of particular importance in patients who are not fully compliant and miss an occasional dose, but would be expected to have less impact on BP with a drug that has a longer and more stable duration of action.
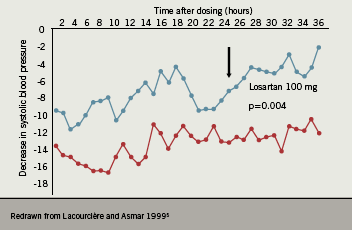
Differences in the magnitude of response between different ARBs have also been evaluated. In 2002, Elmfeldt et al. conducted an analysis of the dose-response relationships for the first four available ARBs: losartan, valsartan, irbesartan and candesartan.8 This investigation demonstrated that differences in efficacy between ARBs do exist, with candesartan showing the greatest dose-related reduction in BP (figure 3).
This study was criticised since only data from disparate studies submitted to the Food and Drug Administration (FDA) for regulatory and licensing purposes were analysed. Therefore, to determine whether the pharmacological characteristics of ARBs made a difference to antihypertensive efficacy, the author undertook a meta-analysis of all studies in which two ARBs, candesartan and losartan, were compared directly in hypertensive patients.9 A systematic literature search of databases from January 1980 to October 2008 identified 13 studies in which candesartan and losartan, either as monotherapy or in fixed combination with hydrochlorothiazide (HCTZ), were compared in randomised trials in hypertensive patients. Data from 4,066 patients were included in the statistical analysis, with mean changes in systolic and diastolic BP being compared for each drug alone, and after stratification for dose and for combination with HCTZ. The results showed a consistent benefit for candesartan, averaging a difference of 3.22 mmHg in systolic BP between the two treatments (figure 4).
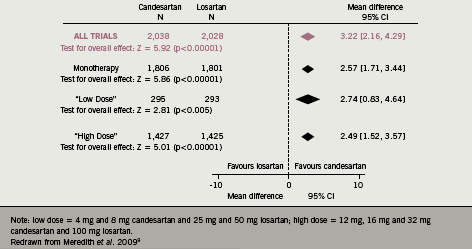
Similar findings were seen for diastolic BP: candesartan monotherapy reduced diastolic BP by an additional 1.8 mmHg compared with losartan, and combination therapy with HCTZ reduced diastolic BP by an additional 4.4 mmHg after candesartan treatment compared to losartan. Although such differences may appear relatively modest, it is known that the overall effect is impressive: using the methodology established by Wald et al.,10 this additional BP response could reduce coronary heart disease events by 32% and reduce stroke by 44%. On the basis of the differential with the combination therapy using HCTZ, similar calculations predict that coronary heart disease events would be reduced by 41% and stroke by 55%.
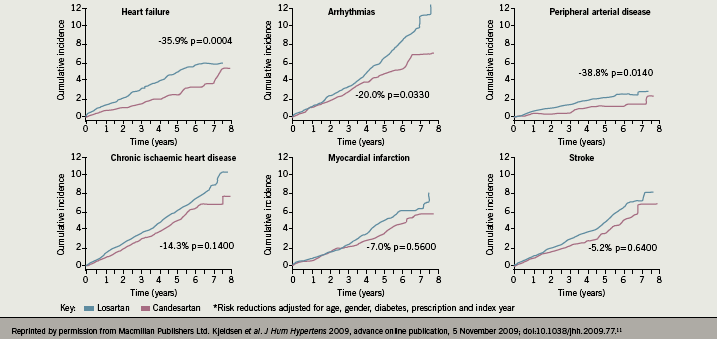
The results of this meta-analysis are further supported by the Real-Life study, in which the hypothesis that losartan and candesartan have different primary preventive effects on cardiovascular risk in patients without known cardiovascular disease was evaluated at 72 primary care centres in Sweden.11 Of the 24,943 eligible patients, in all 14,100 were diagnosed with hypertension and prescribed losartan (n=6,771) or candesartan (n=7,329). The median follow-up was two years, with maximal follow-up of nine years (36,339 patient-years). The observational data suggested that there was no difference in overall BP reduction (mean 145/85 mmHg) when comparing the losartan and candesartan groups during follow-up, although 20% more patients in the losartan group required treatment with a thiazide diuretic to achieve BP control compared to those receiving candesartan. However, the candesartan group had a lower adjusted hazard ratio for total cardiovascular disease (0.86, 95% CI 0.77–0.96, p=0.0062), heart failure (0.64, 95% CI 0.50–0.82, p=0.0004), cardiac arrhythmias (0.80, 95% CI 0.65–0.92, p=0.0330) and peripheral arterial disease (0.61, 95% CI 0.41–0.91, p=0.0140). (Baseline factors of age, gender, diabetes and prescription index year were adjusted in the model.) Since there was no evidence of a differential BP reduction between the two groups, this suggests that other mechanisms related to different pharmacological properties of the drugs may be causing different clinical outcomes (figure 5).
Conclusions
Despite the widespread idea that all ARBs are the same and show a ‘class’ effect, significant pharmacological differences with respect to efficacy and duration of action are apparent within the ARB class. A recent meta-analysis comparing two members of the ARB class, losartan and candesartan, suggests that such differences may translate into differences in BP reduction, and thus a reduction in cardiovascular risk. Observational data also suggest that patients prescribed the long-acting ARB candesartan have considerably better CV outcomes
than those prescribed losartan. Differences do exist between the products within the ARB class, and such differences should be taken into account by the prescribing
physician.
Conflict of interest
PM has received research grants, honoraria for consultancy, advisory board attendance and speaker fees from a number of pharmaceutical companies, including AstraZeneca, Bayer, Boehringer Ingelheim, GSK, MSD, Pfizer and Takeda.
Key messages
- The angiotensin receptor blockers (ARBs) as a class are chemically similar but important pharmacokinetic and pharmacodynamic differences do exist between them
- The ARBs differ in the magnitude and duration of the antihypertensive response: this has important implications in clinical practice
References
- Vauquelin G, Fierens F, Van Liefde I. Long-lasting AT1 receptor binding and protection by candesartan: comparison to other biphenyl-tetrazole sartans. J Hypertens 2006;24:S23–S30.
- Bhuiyan MA, Ishiguro M, Hossain M et al. Binding sites of valsartan, candesartan and losartan with angiotensin II receptor 1 subtype by molecular modeling. Life Sciences 2009;85(3–4): 136–40.
- Belz GG, Fuchs B, Malerczyk C, Magin SG, Roll S, Mutschler E. Inhibition of angiotensin II pressor response and ex vivo angiotensin II radioligand binding by candesartan cilexetil and losartan in healthy human volunteers. J Hum Hypertens 1997;11(Suppl 2):S45–S47.
- Van Liefde I, Vauquelin G. Sartan-AT1 receptor interactions: in vitro evidence for insurmountable antagonism and inverse agonism. Mol Cell Endocrinol 2009;302(2):237–43.
- Lacourcière Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study. Candesartan/Losartan study investigators. Am J Hypertens 1999;12(12 Pt 1-2):1181–7.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903–13.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665.doi:10.1136/bmj.b1665
- Elmfeldt D, Olofsson B, Meredith P. The relationships between dose and antihypertensive effect of four AT1-receptor blockers. Differences in potency and efficacy. Blood Press 2002;11:293–301.
- Meredith PA, Murray LS, McInnes GT. Comparison of the efficacy of candesartan and losartan: a meta-analysis of trials in the treatment of hypertension. J Hum Hypertens 2009;1–7. Advance online publication, 17 December 2009; doi:10.1038/jhh.2009.99.
- Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis of 11 000 participants from 42 trials. Am J Med 2009;122:290–300.
- Kjeldsen SE, Stålhammar J, Hasvold P, Bodegard J, Olsson U, Russell D. Effects of losartan vs. candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens 2010;24:2634–73. Advance online publication, 5 November 2009;
