A report from the 5th Annual Scientific Meeting of the Cardiorenal Forum, held at the Royal College of Physicians, London, October 2010, in association with the Dutch Society of Nephrology and the Netherlands Cardiorenal Group
Introduction
The association between decreased renal function and cardiovascular morbidity and mortality is undeniable. Observational epidemiological studies repeatedly demonstrate a bidirectional, prognostically adverse relationship regardless of whether the primary insult is cardiovascular or renal in origin. Thus one may identify ‘cardiovascular’ pathologies, such as heart failure or hypertension, where adverse prognosis is associated with decreased glomerular filtration rate (GFR) and inappropriate renin-angiotensin-aldosterone activation. Equally, prognosis in renal disease is driven by cardiovascular events. The reader – a nephrologist say, scion of an organ specific culture (embracing nomenclature, training and specialism) – will immediately muse upon whether hypertension or heart failure are not in fact primarily ‘renal’ pathologies. The cardiologist may have other views. That the association exists is not in doubt, but the direction and causality of the relationship remains uncertain.
The dispassionate observer might question current approaches to understanding this interrelationship. Fundamental to dealing with uncertainty is acknowledging the limitation of conventional wisdom. Not knowing, and indeed acknowledging the unknown, is an immutable scientific principle. In addition to this acceptance of the ‘known unknown’ there are also the ‘known knowns’ and the ‘unknown unknowns’ – concepts originally attributable to Nassim Nicholas Taleb, a Professor of Risk Engineering in New York. Importantly, the ‘unknown unknown’ requires that a state of ignorance is embraced and preconceptions abandoned.
The Cardiorenal Forum was formed in 2005 in response to the ‘known unknowns’ of the then recently-named cardiorenal syndrome. The Forum’s 5th Annual Scientific meeting sought to outline and challenge current concepts of the cardiorenal syndrome, to provide insight into ongoing and future research, and to highlight the growing importance of co-operation between the cardological and nephrological communities.
Things we now know we do not know
Anaemia
Anaemia, in both heart failure and chronic disease, is an independent risk factor for morbidity and mortality. Anaemia (haemoglobin <12 g/dL in women and <13 g/dL in men) was present in one out of four patients in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) study programme, and was associated with worse outcome.1 Historically, the treatment of anaemia has been directed to normalisation of the haemoglobin (Hb) level. Erythropoiesis-stimulating agent (ESA) therapies such as erythropoietin (EPO) have been shown to decrease the need for transfusions in haemodialysis patients and improve quality of life.2 More recently, despite no firm trial evidence, their use had extended to patients with heart failure and advanced renal disease.
The wisdom of this approach is now in question. The CREATE (Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta) and CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) studies found no cardiovascular benefit from treating pre-dialysis patients to normal Hb levels.3,4 Of more concern, in the TREAT (Trial to Reduce Cardiovascular Events with Aranesp® Therapy) study of pre-dialysis patients with diabetes, chronic kidney disease (CKD) and anaemia, the use of the ESA darbepoetin alfa did not reduce the risk of death, or that of cardiovascular or renal events, and was associated with an increased risk of stroke.5
The assumption that the adverse association between anaemia and outcome in renal failure translated linearly into a rationale for therapeutic intervention appears misplaced. Anaemia may not be the only determinant of risk, with factors such as iron deficiency and erythropoietin resistance contributing to the disease phenotype. Perhaps the role of erythropoietin, the dose of the drug, the effect of resistance or the optimum level of Hb have been misunderstood or misinterpreted. Correction of iron storage or availability may however be important. Thus intravenous iron increases Hb in CKD,6 and in patients with heart failure it has also been shown to be safe and to improve symptoms and exercise tolerance in the short term.7 Longer-term safety and outcome data is awaited.
Coronary artery disease (CAD) and revascularisation
It would seem reasonable to argue that, since mortality and morbidity from CAD is increased when there is renal impairment, interventions known to improve prognosis in CAD would be of value in patients with kidney disease. The first attempt to explore this hypothesis, using atorvastatin in patients with diabetes undergoing haemodialysis, found no benefit despite substantial reductions in low-density lipoprotein cholesterol (LDL).8 It has been postulated that the idea was correct, but the intervention too late in the disease course.
A more direct exploration of the impact of obstructive CAD is to consider the effect of revascularisation. In CAD, coronary revascularisation is indicated to reduce mortality in acute myocardial infarction (MI), and for symptom control in stable angina. The incidence of CAD in patients with end-stage renal disease (ESRD) is high, with a 45% mortality from cardiovascular events,9 although CAD is often a clinically silent disease in this population and may be more prevalent than data suggest. In CAD, registry data indicate that percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) both show similar long-term mortality and rates of death, Q-wave MI, or stroke. However, PCI with stent insertion was associated with higher rates of repeat revascularisation than CABG.10 It is not known if revascularisation is safer than medical therapy in ESRD, nor if PCI or CABG is preferable. A registry of dialysis patients with significant CAD found that long-term, event-free survival in those managed medically was similar to that of the overall study population, suggesting that revascularisation may not be of benefit in this group.11 A finding in post-hoc analysis that a small subgroup of patients who declined revascularisation were at an increased risk of events and death requires formal study.
In patients with non-haemodialysis CKD and multi-vessel CAD, survival was greater after CABG compared to PCI with drug-eluting stents, but at the expense of a greater short-term risk of requiring permanent haemodialysis, according to recent registry data from Texas.12 So might it then be reasonable to screen the pre-transplant population for CAD? Dr Chris Baker (Imperial College Health Care Trust London) thought not, arguing that angiographic screening in the pre-transplant population will inevitably detect significant disease, leading to coronary intervention in those that may not have needed it and subjecting them to further risk. He highlighted that before embarking on a widespread angiographic screening service, evidence from large multicentre randomised controlled trials (RCT) of angiographic screening and revascularisation in the entire CKD population is warranted.
Renal artery disease and revascularisation
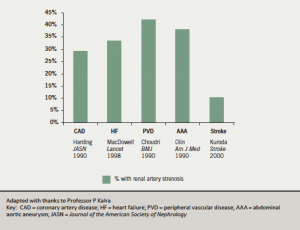
Insofar as renal blood flow is an important determinant of renal function, and the renal artery is a dominant influence in renal perfusion, intervention to improve renal artery flow might be expected to be beneficial. Atherosclerotic renovascular disease (ARVD) is an increasingly important cause of renal failure and is associated with extensive cardiovascular comorbidity (figure 1). In addition, ARVD is present in one third of patients with chronic heart failure and is associated with a three- fold increase in mortality.13, 14 Renovascular disease may first present as acute pulmonary oedema, angina, congestive cardiac failure or acute kidney injury with severe hypertension. A small non-randomised series has reported that renal revascularisation may be beneficial in the setting of acute heart failure, with 92% of those treated remaining free of heart failure during follow-up.13 Similarly, small observational studies report limited benefit from renal revascularisation, in particular improvement in New York Heart Association class or reduced hospitalisations in patients with chronic heart failure. No formal trial data are available.
Renal angioplasty with stent insertion is widely available for the treatment of renal artery stenosis (RAS). Current indications for renal artery revascularisation in ARVD include acute kidney injury requiring dialysis, flash pulmonary oedema, rapidly deteriorating renal function, severe and resistant hypertension and prevention of renal artery obstruction. The STAR randomised controlled study comparing percutaneous renal revascularisation and medical management in ARVD patients found no benefit of revascularisation.15 It was suggested that the study was underpowered with too short a follow up period to detect any significant difference. However the recent, more fully-powered ASTRAL (Angioplasty and Stenting for Renal Artery Lesions) trial in 806 patients with anatomically significant ARVD found that, compared to medical therapy, renal revascularisation was not associated with an improvement in renal function, blood pressure, cardiovascular events or survival.16
How can these findings be reconciled? Despite the lack of benefit of revascularisation in ARVD, Professor Philip Kalra (Salford Royal Hospital Trust and University of Manchester) questioned whether there might be subgroups amenable to adequately powered studies that would benefit from renal revascularisation – for example, patients with chronic heart failure and left ventricular hypertrophy (LVH).
Strategies to improve knowledge
It is evident that much remains to be explained and understood. When a well-designed randomised controlled trial finds an intervention to be of no benefit, providing there is also no harm, subgroup analysis for signals of potential improvement in therapeutic strategy or target group is a common and reasonable response. This is not, however, a substitute for
re-examining the original hypothesis.
Classification
It is less certain whether reclassification and subdivision of the cardiorenal syndrome assists in understanding the processes involved. Dr Chris McIntyre (Derby City General Hospital and University of Nottingham) argued that a classification system should be designed to provide prognostic information, to identify meaningful discrete pathophysiology, to guide treatment and to capture all the dimensions of a disease process. He cautioned that the failure of the recently proposed classification17 to fulfil any of these criteria highlighted both our lack of understanding of the complex bi-directional interaction between heart and kidneys and the allure of ’magic pixies’ – pathophysiological figments of our imagination.
Cardiorenal biomarkers
If, to date, conventional thinking has been thwarted, are there other areas to consider? Perhaps the current concept of renal impairment, in failing to mirror the underlying process, hinders our understanding? Whilst both reduced GFR and albuminuria are important and independent risk factors for mortality and morbidity in patients with chronic heart failure,18,19 the underlying causes of these surrogates of impairment are multifactorial. Although renal hypoperfusion and associated hypoxia seem centrally implicated, does the inference of both glomerular and tubular damage alter the pathophysiolgical behaviour of the disease? Would renal biomarkers which distinguish between differing mechanisms of renal impairment help stratify disease course?
This is an area of great study and promise. Urinary neutrophil gelatinase associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1) and N-acetyl-b-D-glucosaminidase (NAG) are all markers of tubular damage. Both urinary KIM-1 and NAG levels have shown a relationship with plasma NT-proBNP concentrations, while urinary NAG levels are associated with impaired renal function and perfusion. Furthermore increased urinary KIM-1 and NAG levels are associated with an increased risk of death or heart failure hospitalisations, independently of GFR and urinary albumin excretion.20 Research is already underway to investigate whether the prognostic impact of these tubular markers serve as a useful adjunct to GFR, independent of the prevalence of albuminuria.
Arterial function
It is also important to look outward, beyond both the kidney and the heart. Increased arterial stiffness is a hallmark of CKD, and is associated with adverse alterations in cardiac structure and function contributing to subsequent cardiovascular and renal complications. Increased arterial stiffness, measured by pulse wave velocity (PWV) is a risk factor for hypertension and an increase PWV in patients with ESRD is associated with poorer survival rates.21 Greater arterial stiffness is also associated with cardiac hypertrophy and heart failure, particularly in patients with heart failure and preserved ejection fraction (HFpEF). The complex interplay of ventricular loading and longitudinal and radial function was explored by Dr Alan Fraser (Cardiff University). Increased central arterial stiffness may disrupt ventricular-arterial coupling, with slowed diastolic filling causing subendocardial ischaemia, and both ischaemia and elevated afterload leading to impairment in long axis systolic and diastolic function. Emerging therapeutic avenues include the regulation of central artery stiffness through nitric oxide (NO), endothelin-1 (ET-1) and C-type natriuretic peptide. Thus abnormalities of arterial function impact both on renal and cardiac function. Still unexplained, however, is the interaction between heart, vessels and kidney that leads to congestion as part of the heart failure syndrome.
Conclusion
A clear understanding of the complex interplay of cardiovascular and renal impairment remains elusive. The association is clear but the direction and ’language’ through which the systems intercommunicate is not. Interventions to date based on the existing pathological strata of obstructive vasculopathy have failed to improve outcome. So too have interventions directed at anaemia – perhaps itself more a bystander than a contributory pathology. We know more about what we do not know than what we do know. If, as one speaker said, the kidney is “a glorified blood vessel”, is it helpful to talk about the kidney as a singular concept? Would a broader biomarker approach, integrating renal ‘preload’, glomerular function, tubular function and renal ‘afterload’ help? Joint cardiorenal research that reduces the “isolation of medical specialities” is required to explore these ‘unknowns’ and to dispel the temptations of “magic pixies riding on a flake of rainbow unicorns”.
Diary date
The 6th Annual Scientific Meeting of the Cardiorenal Forum will be held on the 7th of October, 2011, at the Royal Society in London. The theme will be ‘Cardiorenal disease in the changing health service.’
For further details see
www.cardiorenalforum.com
Parthipan Sivakumar
Senior House Officer in Cardiology
Hugh McIntyre
Consultant Cardiologist
Queen Alexandra Hospital, Portsmouth, PO6 3LY, and Conquest Hospital, Hastings, East Sussex TN37 7RD
Correspondence to:
References
- O’Meara E, Clayton T, McEntegart MB et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2006;113:986-94.
- Jones M, Ibels L, Schenkel B et al. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int 2004;65:757-67.
- Singh AK, Szczech L, Tang KL et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006;355:2085-98.
- Drueke TB, Locatelli F, Clyne N et al. Normalization of hemoglobin levels in chronic kidney disease and anemia. N Engl J Med 2006;355:2071-84.
- Pfeffer MA, Burdmann EA, Chen CY et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019-32.
- Silverberg DS, Blum M, Agbaria Z et al. The effect of i.v. iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol 2001;55:212-19.
- Okonko DO, Grzeslo A, Witkowski T et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008;51:103–12.
- Wanner C, Krane V, März W et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–48.
- USRDS 2000. Annual data report. 583-689.
- Park DW, Kim YH, Yun SC et al. Long-Term outcomes after stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease. 10-year results of bare-metal stents and 5-year results of drug-eluting stents from the ASAN–MAIN (ASAN Medical Center–Left MAIN Revascularization) Registry. J Am Coll Cardiol 2010;56:1366-75.
- De Lima JJ, Gowdak LH, de Paula FJ et al. Treatment of coronary artery disease in hemodialysis patients evaluated for transplant-a registry study. Transplantation 2010;89:807-8.
- Ashrith G, Lee VV, Elayda MA et al. Short- and long-term outcomes of coronary artery bypass grafting or drug-eluting stent implantation for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol 2010;106:348-53.
- Kalra PA. Renal revascularization for heart failure in patients with atherosclerotic renovascular disease. Nephrol Dial Transplant 2010;25:661-3.
- Kane GC, Xu N, Mistrik E, Roubicek T et al. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant 2010;25:813-20.
- Bax L, Mali WP, Buskens E et al. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003;16:807-12.
- Chrysochou C, Kalra PA. Current management of atherosclerotic renovascular disease – what have we learned from ASTRAL? Nephron Clin Pract 2010;115:c73-c81.
- Ronco C, Haapio M, House AA et al. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527-39.
- Hillege HL, Nitsch D, Pfeffer MA et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006;113:671e8.
- Jackson CE, Solomon SD, Gerstein HC et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009;374:543–50.
- Damman K, Van Veldhuisen DJ, Navis G et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010;96:1297-1302.
- Blacher J, Guerin AP, Pannier B. Impact of Aortic Stiffness on Survival in End-Stage Renal Disease. Circulation 1999;99:2434-9.
