Little is currently known of the effect of switching statin therapy on cardiovascular outcomes. Using The Health Improvement Network database, patients who had received atorvastatin for ≥ six months were identified. They were classified as ‘switch’ if they were subsequently switched to simvastatin, and were matched to up to four ‘control’ patients who remained on atorastatin. Time to death or first major cardiovascular event was compared, controlling for the matching co-variates, prior statin exposure and baseline cholesterol concentration.
A total of 2,511 switch patients and 9,009 controls were identified. The risk of death or first major cardiovascular event was significantly associated with switching therapy (hazard ratio = 1.30, 95% confidence interval: 1.02–1.64) compared with patients who did not switch. Major cardiovascular events and stroke were also significantly associated with switching. There was no significant difference in all-cause mortality.
While recognising the observational nature of database research, this study has highlighted the potential for poorer cardiovascular outcomes in patients switching statin therapy, compared with patients maintained on their current treatment. This raises concerns for policies that encourage widespread statin switching without careful consideration of individual patient circumstances and cardiovascular risk, and highlights the need for formal trials to assess the consequences of switching statin treatment.
Introduction
Widespread switching of statin therapy to generic alternatives has been advocated to help alleviate some of the financial pressure in the National Health Service (NHS).1 However, little is currently known of the effect on cardiovascular outcomes of switching patients established on cholesterol- lowering therapy to another agent in the same class.
One of the earliest switch studies took place in New Zealand2 when widespread switching from simvastatin to the less potent fluvastatin occurred for economic reasons without an attempt to maintain lipid-lowering efficacy. An audit of 126 patients with established atherosclerotic disease revealed a significant rise in cholesterol levels in 94% of the population and a three-fold increase in thrombotic vascular events (hospitalisations for myocardial infarction [MI], stroke, unstable angina or acute limb ischaemia) over a six-month period in comparison to the six-month period prior to the switch.
A UK secondary care study3 audited cardiovascular outcomes in patients admitted with acute coronary syndromes. The first cohort of patients (n=100) admitted to hospital in 2005 with MI or unstable angina were treated with atorvastatin 40–80 mg and the second cohort (n=121) in 2006 were treated with simvastatin, predominantly at the 40 mg dose. Outcomes during follow-up ranging from three to six months demonstrated a significant increase in mortality (17% versus 5%) and a significant increase in cardiac re-admissions (53 versus 33) in the cohort treated with simvastatin compared with the atorvastatin-treated cohort.
A UK primary care audit4 examined patients switching from atorvastatin 10–20 mg to simvastatin 20–40 mg in a single general practice. The authors went to considerable effort to ensure that individuals who were not appropriate for switch were excluded. In total, 122 patients were screened and 52 (43%) were deemed to be ineligible for the switch. The majority of these exclusions were due to inadequate cholesterol control at baseline, prior intolerance to simvastatin or a history of previous simvastatin use that had failed to reduce cholesterol to target.
Of the remaining 70 patients, six patients (8.6%) were above target cholesterol post switch, one patient (1.4%) switched back due to simvastatin intolerance and eight patients (11.4%) did not return for follow-up cholesterol testing. Where cholesterol status was recorded the change was not statistically significant.
The present database study explores the impact of switching statins in a UK general practice setting. The population studied is considerably larger than is possible in any individual practice and this therefore allows the impact of switching to be assessed in terms of cardiovascular outcomes.
Materials and methods
This is an observational study within UK general practice using The Health Improvement Network database. This NHS database contains anonymised primary care data and can be used by academic, commercial and other research organisations to undertake studies of epidemiology, drug safety, and disease prevention and treatment. Research studies for publication conducted using this database are approved by a nationally accredited ethics committee. At the time of analysis, the database contained data from 300 practices across the UK with 4.77 million patients. The study was conducted according to a pre-defined protocol and ethical approval was obtained. The primary objective of the study was to compare cardiovascular outcomes in patients who were switched from atorvastatin to simvastatin with those in patients who remained on atorvastatin. Secondary objectives were to examine effects on lipid parameters, achievement of the UK Quality Outcomes Framework (QOF) targets5 and adherence to statin therapy after switching.
Patients receiving atorvastatin for at least six months were identified in the database. The study was a cohort design with patients selected according to drug treatment and not outcomes. The investigators were unaware of outcomes during patient selection. Within the cohort patients were classified as ‘switch’ if
they were subsequently switched to simvastatin, and were matched to up to four ‘control’ patients who remained on atorvastatin. The matching co-variates were gender, general practitioner (GP) practice, history of MI, diabetes and time since last statin exposure. The date of switching was the ‘index’ date in the switch cohort; the date of the first atorvastatin prescription after this time point was the ‘index’ date for their respective controls. Index dates ranged from October 1997 to May 2005 for switch patients and from December 1997 to June 2005 for controls.
The primary outcome measure was time to death or first major cardiovascular event defined as MI, stroke or coronary revascularisation. Hazard rates were reported for each cohort and hazard ratios (HRs), with 95% confidence intervals (CI) calculated using a Cox proportional hazards model and a significance level of p<0.05. HRs were also reported for the individual components of the primary outcome. The data were adjusted for age, baseline (pre-index) total cholesterol (TC) concentration, prior cumulative statin exposure and the matching co-variates (gender, history of MI, diabetes and time since last statin exposure).
Discontinuation from assigned therapy (defined as at least 90 days’ non-exposure) was assessed after the index date for the switch and control groups. Time to discontinuation was analysed and HRs for discontinuation reported, with adjustment for the matching co-variates and prior cumulative statin exposure. Analysis of outcomes was censored at the point of treatment discontinuation.
TC and low-density lipoprotein (LDL) cholesterol concentrations were documented where recorded in the database. All patients for whom cardiovascular outcomes were assessed had a pre-index (baseline) cholesterol measurement. In a smaller subgroup of patients a set of three lipid values were available (statin naïve, pre-index and one-year post-index). In this subgroup the percentage of patients achieving the QOF TC target < 5.0 mmol/L at one year was recorded.
Results
Within the database 3,272 switch patients and 10,995 matched controls were identified. Of these, a recorded baseline (pre-index) cholesterol measurement was available for 2,511 switch patients and 9,009 controls and these subjects were assessed for cardiovascular outcomes. The mean baseline TC concentration was 5.2 mmol/L prior to switching. This was significantly higher (p<0.001) than the corresponding pre-index level of 4.7 mmol/L in the matched control group (table 1). The analysis for cardiovascular outcomes was adjusted for this difference in baseline cholesterol.
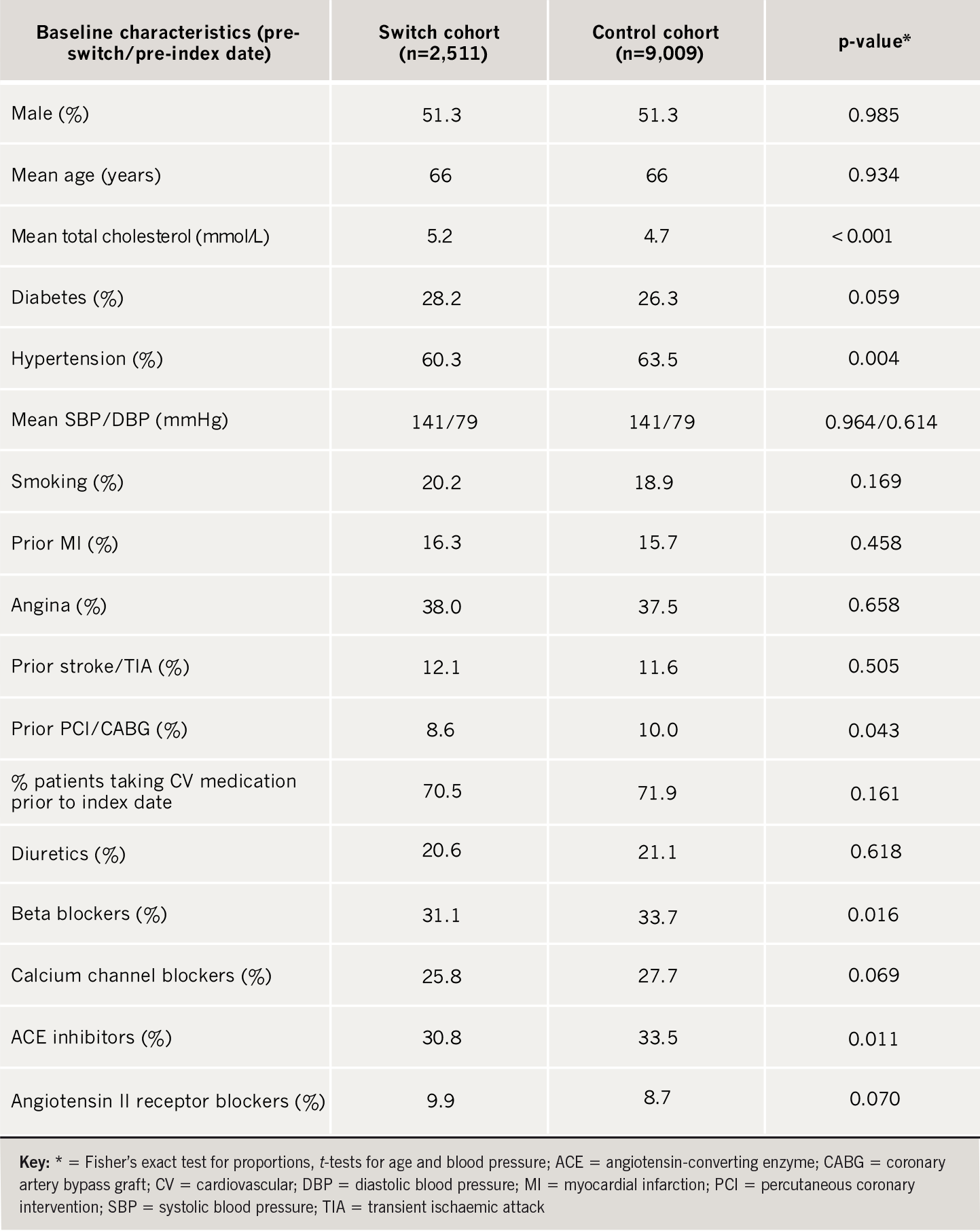
The switch and control cohorts were well matched for age, gender, history of MI/angina, diabetes, smoking and overall cardiovascular medications (table 1). Slightly more patients in the control group were taking beta blockers/angiotensin-converting enzyme (ACE) inhibitors, had a previous percutaneous coronary intervention (PCI)/coronary artery bypass graft (CABG) and had a history of hypertension (although mean blood pressure was the same in each group).
Median follow-up was 1.2 years. Some 24.5% of the switches took place during the period 1997–2002 and 75.5% of the switches took place in the period 2003–2005. Prior to switching 93.2% of patients were taking atorvastatin 10–20 mg (70.8% on 10 mg and 22.4% on 20 mg), with 6.0% taking atorvastatin 40 mg and 0.8% taking atorvastatin 80 mg. After switching 70.5% of patients were taking simvastatin 20–40 mg (39.4% on 20 mg and 31.1% on 40 mg), with 25.9% taking simvastatin 10 mg and 3.5% taking simvastatin 80 mg. In the control group 82.2% of patients were taking atorvastatin 10–20 mg (58.5% on 10 mg and 23.7% on 20 mg) with 15.0% taking atorvastatin 40 mg and 2.8% taking atorvastatin 80 mg after the index date.
Primary outcome measure
Cardiovascular outcomes in the two groups are shown in table 2, with data adjusted for the mean baseline cholesterol concentration, as well as for the matching co-variates, age and prior cumulative statin exposure.

The risk of death or first major cardiovascular event, defined as MI, stroke or coronary revascularisation, was significantly associated with switching statin therapy (HR = 1.30, 95%CI: 1.02–1.64) compared with controls. There were also significant associations with major cardiovascular events (HR = 1.43, 95%CI: 1.10–1.87) and with stroke (HR = 2.14, 95%CI: 1.21–3.81) independently. There was no significant difference in MI, revascularisation or all-cause mortality. Figure 1 shows the time to death or first major cardiovascular event using the Kaplan-Meier method.

Discontinuation rates
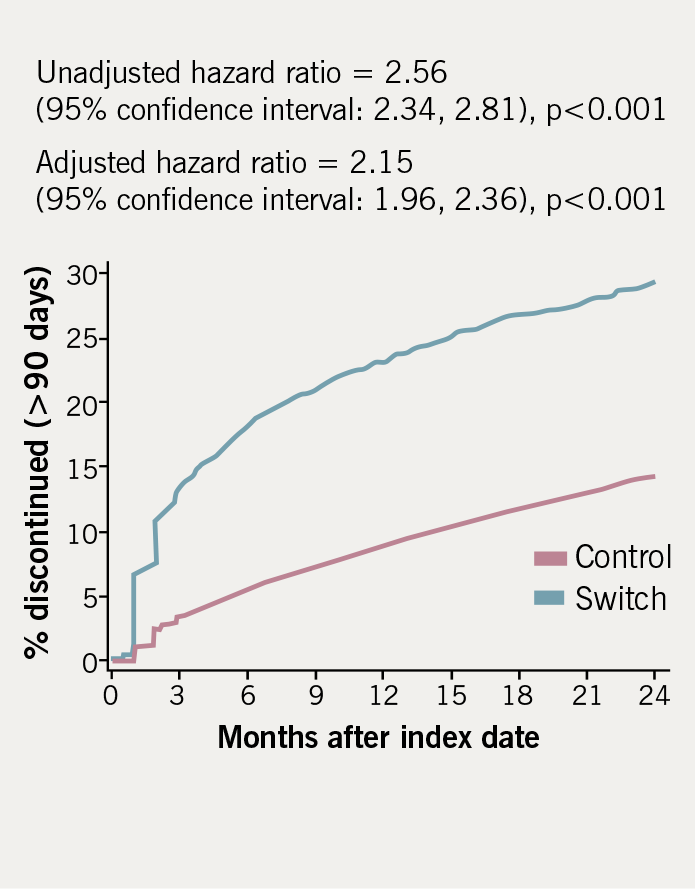
Discontinuation rates (defined as at least 90 days non-exposure) for the switch and control groups are demonstrated in table 3. Discontinuation rates in the switch cohort were more than twice those in the controls after adjusting for co-variates (adjusted HR = 2.15, p<0.001). Figure 2 shows the time to discontinuation using the Kaplan-Meier method.

Lipid changes
Lipid changes were assessed in a smaller cohort of patients in whom three sets of lipid measurements were available (statin naïve, baseline pre-switch [pre-index] and one-year post-switch [post-index]). TC measurements were available for 1,257 switch patients and 4,792 controls (approximately 50% of the study population). LDL-cholesterol measurements were available for 350 switch patients and 1,392 controls (approximately 15% of the study population). Statin naïve lipid levels were similar in both groups (TC 6.5 mmol/L, LDL-cholesterol 4.2 mmol/L).
Prior to switching, this subgroup of patients had a mean TC of 4.9 mmol/L and LDL-cholesterol of 2.7 mmol/L. One-year post-switch, the mean TC was 4.8 mmol/L and LDL-cholesterol was 2.5 mmol/L (p<0.001 vs. baseline). In the control group, the mean TC was 4.6 mmol/L and LDL-cholesterol was 2.4 mmol/L at baseline and one year later (p=non-significant vs. baseline).
Achievement of QOF targets
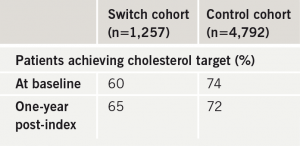
In the UK, GP performance in lipid management is measured by achievement of QOF targets.5 For cholesterol lowering the goal is at least 70% of treated patients achieving a target TC < 5.0 mmol/L. Table 4 shows the percentage of patients achieving the QOF cholesterol target in the switch and control cohorts. At baseline 60% of switch patients were at target TC < 5.0 mmol/L compared with 74% of the control group. At one year 65% of switch patients were at target compared with 72% of controls. Although improvement was demonstrated in the switch group, overall target achievement still fell short of the QOF 70% threshold.
Discussion
In this analysis the risk of death or major cardiovascular events was significantly associated with patients switching statin therapy compared with controls who did not switch. This finding could relate to a number of factors. Although the two groups were matched as closely as possible and contained a substantial number of patients, the possibility of unrecognised confounding cannot be excluded. However, the matching at baseline appears to have produced two cohorts with a similar cardiovascular risk profile and the adjusted analyses attempt to address any residual imbalance as far as is feasible in a database study. The difference in baseline cholesterol concentration between the two groups was an important confounding factor but after adjustment for this parameter a significant difference in cardiovascular outcomes remained.
Patients who switched therapy discontinued at a significantly higher rate compared with patients who did not switch. Outcomes were censored at the point of treatment discontinuation and so the difference in outcomes cannot be explained by discontinuation of statin therapy. This may have underestimated the difference in outcomes given that lipid control may have deteriorated in the patients discontinuing treatment. Poor compliance after a change in statin treatment has been reported previously6 and it highlights the importance of close patient follow-up and monitoring after a switch to ensure adherence and maintenance of lipid control.
Reasons for switching were not recorded in the database. Some switching may have been done for medical reasons related to efficacy, tolerability or compliance. It is important to note, however, that over 75% of switching in this analysis occurred during the period 2003–2005. In some of these patients switching may have occurred for financial reasons related to the availability of generic simvastatin in the UK in 2003, followed by significant price reductions for simvastatin
20 mg in early 2004 and simvastatin 40 mg in late 2004/early 2005.
The lipid subgroup analysis did not demonstrate a deterioration in cholesterol control in the switch group that could explain the increase in cardiovascular events. However, it is important to remember that this subgroup included only about 50% of patients (for TC measurements) and 15% of patients (for LDL-cholesterol measurements) in whom cardiovascular outcomes were assessed and so may not be reflective of lipid changes in the total population. The remaining patients with missing cholesterol data may have demonstrated poorer lipid control. Inclusion of these patients’ lipid values may have revealed a deterioration in cholesterol control in the switch group, which may have accounted for the difference in outcomes.
Differences between statins independent of cholesterol lowering cannot be excluded, such as effects on inflammation, plaque stabilisation and endothelial function.7 A number of randomised trials using atorvastatin have consistently demonstrated an early time to benefit,7–11 which may relate to non-lipid- lowering effects. This has been observed in a broad spectrum of patient populations including those with acute coronary syndromes,8 stable coronary heart disease,9 diabetes10 and hypertension,11 and outcomes studies have been halted earlier than anticipated due to the observed benefits.12–13 These findings raise the hypothesis that switching from atorvastatin to simvastatin may have adverse consequences even if
lipid control is maintained but this requires further study.
Current financial pressures in the NHS are driving widespread statin switching policies to achieve cost savings. At a time when the burden of cardiovascular disease is falling, population statin switching that does not focus on the individual patient could result in more cardiovascular events, hospital admissions and additional costs for the NHS in the longer term. Each patient should be assessed on a case-by-case basis to determine whether a switch in statin therapy is clinically appropriate. The National Institute for Health and Clinical Excellence (NICE) TA094 statin appraisal14 discusses the importance of individualisation of statin prescribing for initial therapy and this would appear to be just as important for any proposed change in therapy.
Study limitations
This is an observational database study and as such has recognised limitations. The findings should therefore be regarded as hypothesis generating. The results may have been influenced by confounding factors although the two groups appeared to be well matched. The study protocol was not designed to evaluate the reasons why patients were switched and was not powered for detailed subgroup analysis or to assess particular dosage switches from atorvastatin to simvastatin. Practitioners may have monitored patients switching treatment more closely but restricting the analysis to the more definitive outcomes of death, MI, stroke and revascularisation is likely to have minimised any detection bias.
Conclusions
This study has highlighted, in a real-world primary-care setting, the potential for poorer cardiovascular outcomes associated with switching statin therapy compared with patients who do not switch. This raises concerns for policies that encourage switching of statin therapy in routine clinical practice without careful consideration of individual patient circumstances and cardiovascular risk. The study has well-recognised limitations but highlights the need for formal trials to assess the consequences of switching statin treatment.
Acknowledgements
No content development support was provided but assistance in formatting the figures and in preparing the manuscript for submission was provided by Envision Pharma and funded by Pfizer Inc.
Conflict of interest
At the time of this analysis all authors were either employees of Pfizer or were working on behalf of Pfizer.
Key messages
- Little is currently known of the effect of switching statin therapy on cardiovascular outcomes
- While recognising the observational nature of database research, this study has highlighted the potential for poorer cardiovascular outcomes in patients switching statin therapy, compared with patients maintained on their current treatment
- This raises concerns for policies that encourage widespread statin switching without careful clinical assessment of individual patients and highlights the need for formal trials to assess the consequences of switching statin treatment
- The additional finding of poorer adherence to therapy after changing treatment highlights the importance of close monitoring of patients and auditing of switch programmes to check for compliance, cholesterol control and, most importantly, cardiovascular outcomes
References
- Moon JC, Bogle RG. Switching statins. BMJ 2006;332:1344–5.
- Thomas M, Mann J. Increased thrombotic vascular events after change of statin. Lancet 1998;352:1830–1.
- Butler R, Wainwright J. Cholesterol lowering in patients with CHD and metabolic syndrome. Lancet 2007;369:27.
- Usher-Smith JA, Ramsbottom T, Pearmain H, Kirby M. Evaluation of the cost savings and clinical outcomes of switching patients from atorvastatin to simvastatin and losartan to candesartan in a primary care setting. Int J Clin Pract 2007;61:15–23.
- General Medical Services contract. Available from: http://www.nhsemployers.org/primary/ primary-886.cfm. Accessed 28th March, 2007.
- Thiebaud P, Patel BV, Nichol MB, Berenbeim DM. The effect of switching on compliance and persistence: the case of statin treatment. Am J Manag Care 2005;11:670–4.
- Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol 2005;96(suppl):54F–60F.
- Ray KK, Cannon CP, McCabe CH et al. Early and late benefits of high dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005;46:1405–10.
- LaRosa JC, Grundy SM, Waters DD. Intensive lipid lowering with atorvastatin provides early and sustained benefit in patients with stable coronary disease: a secondary analysis of the Treating to New Targets (TNT) study. Circulation 2006;114:796.
- Colhoun HM, Betteridge DJ, Durrington PN et al. Rapid emergence of effect of atorvastatin on cardiovascular outcomes in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2005;48:2482–5.
- Sever PS, Poulter NR, Dahlöf B et al. Different time course for prevention of coronary and stroke events by atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA). Am J Cardiol 2005;96(suppl):39F–44F.
- Colhoun HM, Betteridge DJ, Durrington PN et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
- Sever PS, Dahlöf B, Poulter NR et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
- National Institute for Health and Clinical Excellence. Statins for the prevention of cardiovascular events (TA094). Available from: http://www.nice.org.uk/page.aspx?o=TA094guidance. Accessed 28th March, 2007.
