A new stent which may promote healing of the artery better than currently available drug-eluting stents has shown promising results in initial clinical trials. The Genous Bio-engineered R StentTM, being developed by OrbusNeich, is coated with an antibody to capture a patient’s endothelial progenitor cells, which form a layer over the stent to provide protection against thrombus and minimise restenosis.
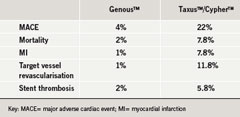
In the current study, the GenousTM stent was associated with significantly fewer major adverse cardiac events (MACE) than the TaxusTMor CypherTM drug-eluting stents. The study, presented at the recent Italian Society of Invasive Cardiology by Dr Federico Piscione (Federico II University of Naples, Italy) involved 195 high-risk patients who received either a GenousTM, TaxusTM or CypherTM stent. Dual antiplatelet therapy was given for one month to the patients receiving a GenousTM stent and for nine months to those given one of the drug-eluting stents.
“GenousTM is a viable alternative to drug-eluting stents, which have raised many safety concerns among the interventional cardiology community,” Dr Piscione concluded.
A large phase III trial of the GenousTM stent is now underway.
