The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) showed that addition of eplerenone to optimal medical therapy reduced morbidity and mortality in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure. This international study also showed that the addition of eplerenone reduced the number and duration of rehospitalisations for heart failure. A budget impact model has been developed to estimate the effect of adding eplerenone to standard care in the UK. The model is based on the results of the EPHESUS study, UK epidemiological data, UK drug acquisition costs and National Health Service (NHS) hospital in-patient costs and average length of stay for England. All costs are expressed in pounds sterling.
It estimates the incremental costs and benefits of adding eplerenone to standard care in heart failure resulting from myocardial infarction, from the perspective of NHS healthcare decision makers over a three-year period.
The model shows that if all eligible patients are treated with eplerenone the estimated cost per life year saved is £6,730 in year three. In a primary care trust with a population of 250,000, this level of treatment results in a reduction of 46 bed days for rehospitalisations due to heart failure, at a cost per bed day avoided of £1,469. With hospital in-patient care the biggest single healthcare cost in heart failure, reduction in hospitalisation is a key priority within the NHS in the UK. Models such as the one described here enable the budgetary consequences of using a new drug to be identified and clarify the role of drug treatment in delivering NHS priorities.
Introduction
The total direct medical costs of heart failure in the UK each year are estimated to be approximately £716 million, with hospital in-patient care the biggest single healthcare cost, accounting for approximately 70% of the total cost of heart failure.1 Budget impact analyses are increasingly being used to complement economic evaluations as they enable healthcare purchasers to examine the relationship between maximised efficiency and affordability.2 This paper describes a budget impact model that estimates the incremental costs and benefits of adding eplerenone to standard care for heart failure resulting from myocardial infarction (MI) over a three-year period, from the perspective of National Health Service (NHS) healthcare decision makers. The model allows the impact of the drug to be measured appropriately with opportunities to quantify both costs and benefits on the total cost of healthcare for heart failure within the UK.
Eplerenone is a selective aldosterone receptor antagonist (SARA), which antagonises aldosterone at the mineralocorticoid receptor without the therapeutic limitations of binding to glucocorticoid, progesterone, or androgen receptors. Eplerenone is indicated, in addition to standard therapy including beta blockers, to reduce the risk of cardiovascular mortality and morbidity in stable patients with left ventricular dysfunction (left ventricular ejection fraction [LVEF] 40% or less) and clinical evidence of heart failure after recent MI.3 The international Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) demonstrated that eplerenone significantly reduced mortality and morbidity in people with left ventricular dysfunction and heart failure post-MI when added to standard therapy.4 Furthermore, eplerenone reduced the number4 and duration5 of rehospitalisations for heart failure.
Methods
The budget impact model was constructed as an Excel® spreadsheet and estimates the incremental costs and benefits of adding eplerenone to standard care during the first three years of use in the treatment of patients with signs of heart failure following MI in the UK. The model was developed for use by both commissioning bodies (e.g. primary care trusts [PCTs]) and secondary care organisations (e.g. drug and therapeutic committees of hospital trusts). The model was developed as a series of linked spreadsheets, to be completely transparent and allow users to view all calculations and to follow the progression of a cohort of initially hospitalised patients with symptoms of heart failure following MI.
Population data from the Office of National Statistics6 and NHS data for England7 and Scotland8 were used to estimate the incidence of hospitalised MI and to populate the baseline case in the model. In the NHS in England in the year 2005–2006, there were 88,003 finished consultant episodes of MI. This equates to approximately 433 episodes per 250,000 population. The rate in Scotland was approximately 524 per 250,000 in 2006.8 Information on in-patient cost and average length of stay was extracted from NHS sources for England 2005–2006.7 Data were weighted by elective and non-elective health-related group episodes to obtain an average cost and length of stay for both MI and heart failure admissions.
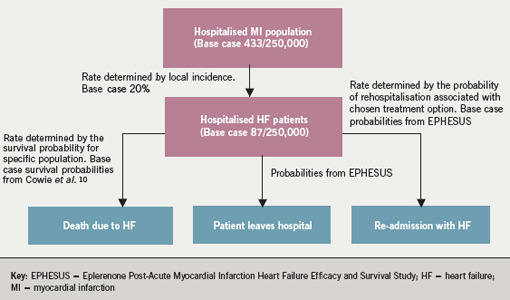
Baseline patient numbers within the model are constructed from the monthly number of episodes of MI for the population under consideration. The National Institute for Health and Clinical Excellence (NICE) has estimated that 20% of hospitalised MI patients show clinical signs of heart failure; thus 20% of the population incident rate is used as the base case within the model.9 In England this equates to 87 patients per 250,000 population entering the model each year. A new cohort of seven patients enters the model each month; the model is unable to account for seasonality due to paucity of relevant data. As each monthly cohort progresses through the model it is subject to a variable monthly probability of death, which is dependent on the longevity of the cohort within the model. The method of calculating this is described below; however figure 1 shows how patient numbers build up within the model.
There is no available epidemiological information on long-term survival in patients developing heart failure as a result of MI. Survival data from clinical trials is likely to be affected by the study design and inclusion and exclusion criteria, and therefore is not suitable for use in a population-based model. The survival probabilities used for this budget impact model are derived from an epidemiological study of all–cause heart failure carried out in West London.10Monthly probabilities of survival within the model are calculated from observed survival data at one, three, six, 12 and 18 months. These data are also extrapolated to calculate probabilities of death on a monthly basis to 36 months, the time period over which the model is run. With the model based on a three-year study, this time horizon is appropriate and accommodates budget-planning cycles within the NHS. Although patients with heart failure due to MI are likely to be younger than those developing heart failure due to other causes and could therefore be considered to have a better prognosis, outcomes from the Global Registry of Acute Coronary Events (GRACE) registry11 indicate that these younger patients developing heart failure post-MI are at a higher risk of death than older patients. To overcome the limitations of data extrapolation based on all-cause heart failure, sensitivity analyses were carried out to investigate the effects of increasing the mortality rate. These have been carried out by reducing the known survival probabilities by 10%, 15% and 20% and recalculating the monthly survival probabilities and hence model outputs for the baseline case of standard therapy.
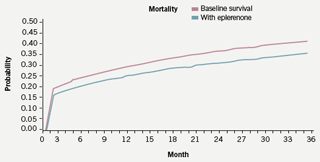
Information on treatment-related reductions in mortality and morbidity used in the model are derived from EPHESUS,4 and applied to UK population data. Probabilities derived from EPHESUS on the reduction in overall mortality are calculated on a monthly basis and applied to base case survival probabilities from month one to month 16, which was the average duration of follow-up within the trial. After month 16 the survival curves are modelled to run parallel to month 36. The rationale for the parallel progression of curves within the model is the reduced rate of cardiac remodelling following MI in treated patients. This reduction in cardiac damage should provide a continuing survival benefit within the timescale of the model. The parallel progression of the curves shown in figure 2 does not, however, indicate any increasing benefit over and above that seen in the EPHESUS study.
Rates of rehospitalisation for heart failure are calculated on a monthly basis from the absolute rates reported in EPHESUS. These rates are then applied on a monthly basis across the time period of the model. Once again the rationale for extrapolation beyond the time period of the trial being that eplerenone-treated patients are subject to less remodelling of cardiac tissue post-MI.
The bed days avoided was calculated from the difference between the baseline bed days and bed days with eplerenone. The baseline bed days were derived from NHS data on admissions for MI and heart failure in England (2005–2006).7 The eplerenone bed days were calculated from the reduction in both re-admissions from baseline seen in the EPHESUS study and a reduction in bed days per admission of 1.7 days.5
The life years gained was calculated from dividing the difference in the numbers of patient months from the model by 12. The incremental cost-effectiveness ratio was subsequently derived by dividing the cost of eplerenone by the survival difference.
Within the model, the population, incidence of MI, and proportion of patients developing heart failure post-MI can be customised; the percentage of eligible patients prescribed eplerenone can be varied on a yearly basis. No option exists to modify model parameters based on clinical trial data at this point in time, but this could be developed.
Only incremental changes in resource use are included in the model. Eplerenone is an addition to standard therapy and not a substitute for alternative treatment options. It is assumed that all patients will be prescribed standard therapy of angiotensin-converting enzyme (ACE) inhibitors, beta blocker, statin, etc. post-MI and that the cost of eplerenone will be the only additional drug cost. Annual drug costs for eplerenone are approximately £555.12 Resource savings are based on reductions in rehospitalisations calculated from those observed in the EPHESUS study, as was the reduced length of stay for rehospitalisation.
Results
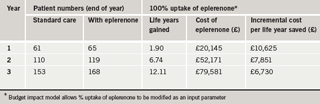
The outputs of the model reported as a comparison of the addition of eplerenone to standard care with standard care alone, as shown in tables 1 and 2, include:
- reduction in mortality
- cost of eplerenone therapy
- incremental cost per life year saved
- reduction in rehospitalisation for heart failure and length of stay
- incremental cost per bed day avoided.
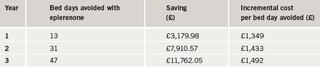
The estimated incremental cost per life year saved if all eligible patients are treated with eplerenone over a three-year period is £6,730 in year three. In a population of 250,000 this level of treatment results in a reduction of 47 bed days for rehospitalisations due to heart failure in the treated population, at a cost per bed day avoided of £1,492.
The Cowie10 study reports survival in all-cause heart failure patients to 18 months; these data were extrapolated to three years for the model. The extrapolation predicts a 48% three-year survival. The sensitivity analyses described above, where the monthly probability of mortality is varied showed that an increased mortality rate is associated with an increased cost per life year saved.

The results of these sensitivity analyses, the estimates of cost per life year saved in year three and the corresponding reduction in rehospitalisation are shown in table 3.
Discussion
Improving the care of people with long-term conditions is a key priority for the NHS, and a central part of the UK Department of Health’s Improvement Plan.13 Just 2% of patients with chronic conditions account for 30% of unplanned hospital admissions.11 The prevention of unplanned admissions and hospital stays has been a recurrent theme in recent NHS policy and there is considerable pressure to reduce costly time spent in hospital.14
NICE estimated in 2003 that heart failure accounts for a total of one million in-patient bed days, accounting for 2% of all NHS in-patient bed days and 5% of all emergency medical admissions to hospital.1 Hospital admissions due to heart failure are projected to rise by 50% over the next 25 years, largely due to the ageing of the population and improved diagnosis and treatment for cardiac events resulting in improved survival. NICE estimate the total annual cost of heart failure to the NHS at £716 million, which is around 1.8% of the total NHS budget; approximately 70% of this total is due to the costs of hospitalisation.1 Although the model described here is focused on assessing direct costs in the NHS, the considerable financial and personal impact of heart failure on other parties including social services, the benefits system, patients and their families and caregivers is recognised.
The model described here provides commissioners of care, and those providing primary and secondary care, with a tool to assess the impact of eplerenone on their local population. The model suggests that adding eplerenone to standard therapy in patients with clinical heart failure post-MI is cost-effective; in particular, eplerenone can reduce the number and duration of rehospitalisations and associated costs.
The major sources of uncertainty within this model include: the uptake of eplerenone; the proportion of MI patients showing signs of heart failure; and the long-term survival of patients. Formal sensitivity analyses, such as those performed on the mortality rate in this evaluation, can be used to assess the impact of uncertainty on the results from economic models; however, this simple model allows the budgetary consequences of different uptake scenarios and varied proportions of MI patients showing signs of heart failure to be readily examined.
Although recent publications have discussed the limitations of absolute thresholds for judging the acceptability of interventions in healthcare,15 in terms of decision making it is generally agreed that definitions from the World Bank16and NICE provide reasonable guides. The World Bank suggest interventions are cost-effective if the life year saved is less than the per capita gross national product (GNP) and within the UK interventions with a cost per quality-adjusted life year (QALY) below £30,000 appear to receive a positive recommendation when assessed by NICE.16 The cost per life year saved estimated for eplerenone of £6,730 in year three can therefore be considered favourably. A similar conclusion to this UK analysis has recently been shown within a US setting, where eplerenone was shown to be cost-effective in increasing years of life when assessed by commonly used criteria.17
One potential limitation of this model may be the use of data derived from a multi-centre, multi-national study as applied to a UK health context. It is possible that triggers for admission and duration of time in hospital vary between countries. However, given the reasons for hospital admission in EPHESUS (mostly acute MI and heart failure), it is likely that community-based treatment would not be appropriate and that hospital-based care would be similar. In particular, analysis of the different geographical regions covered in the study showed no significant difference in length of stay for rehospitalised patients between Europe and the total study population.5 However, recognition that any intervention capable of reducing even a small percentage of adverse outcomes (particularly hospital admission and length of stay) is likely to produce significant cost savings in the management of heart failure, has recently been published.18
When making decisions on the formulary inclusion of new drugs, the effect on all relevant healthcare budgets needs to be considered along with the impact on societal costs and the personal and financial burden on individuals. Models such as the one described here help commissioners and healthcare providers assess the potential costs and benefits that new drugs can accrue and allow them to place the cost of a drug in the context of overall management and treatment. Modelling encourages a broader view of a drug intervention to be identified by quantifying other key factors besides drug budgets. The role of drug treatment to deliver NHS priorities and potentially reduce overall costs in the NHS can thus be readily assessed in local settings.
Conflict of interest
This research was carried out with financial support from Pfizer Limited and there are no conflicts of interest to declare.
MD recieved an unrestricted educational grant from Pfizer UK to help develop and write this paper. He has no other conflicts of interest to declare. MT was employed by Pfizer Ltd at the time of developing the model and writing the manuscript.
Note
The budget impact model described in this paper can be made available by contacting Jason Miller, Clinical Effectivenss Consultant, Pfizer Ltd. Email: [email protected]
Key messages
- Total direct medical costs of heart failure are estimated to be approximately £716 million per year for the UK
- Approximately 70% of the total cost of heart failure is attributable to hospital in-patient care
- Eplerenone has been shown in a randomised controlled trial to significantly reduce cardiovascular mortality and morbidity in people with left ventricular dysfunction and heart failure post-MI, when added to standard therapy
- In particular, eplerenone reduces the number, and duration, of rehospitalisations for heart failure
- This paper describes a budget impact model that can be used by primary care organisations and trusts to estimate the health economic impact of eplerenone in their health community
- Such economic modelling suggests that adding eplerenone to standard therapy in patients with clinical heart failure post-MI can be highly cost-effective, as it costs less than £7,000 for each life year saved
- Using eplerenone in the treatment of heart failure should reduce the burden of heart failure, both on patients and the National Health Service
References
- National Institute for Health and Clinical Excellence. NICE Guideline Number 5. Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: NICE, October 2003. Full guideline, available from: www.nice.org.uk [accessed 17/01/08].
- Trueman P, Drummond M, Hutton J. Developing guidance for budget impact analysis. Pharmacoeconomics 2001;19:609–21.
- Inspra (eplerenone) Summary of Product Characteristics. Pfizer Ltd., 2007.
- Pitt B, Remme W, Zannad F et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–21.
- Gheorghiade M, Pitt B, Chu T-C, Patni R. Length of stay for heart failure hospitalisation in the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Eur J Heart Failure 2004;3(suppl 1):115, Abstract 455.
- Office of National Statistics. National Population Projections. Available from:http://www.statistics.gov.uk/cci/nugget.asp?id=1352 [accessed 15/01/08].
- Department of Health. NHS reference costs 2005-2006, Appendix 4. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_062884 [accessed 15/01/08].
- ISD Scotland. Scottish health statistics. Available from: http://www.isdscotland.org/isd/information-and-statistics.jsp?pContentID=3075&p_applic=CCC&p_service=Content.show& Table IC2 [accessed 17/01/08].
- National Institute for Health and Clinical Excellence. Costing Report NICE clinical guideline 48. MI: secondary prevention. London: NICE, May 2007. Available from: www.nice.org.uk [accessed 17/01/08].
- Cowie MR, Wood DA, Coats AJ et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 2000;83:505–10.
- Steg PG, Dabbous OH, Feldman LJ et al.; for the GRACE investigators. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004;109:494–9.
- Haymarket Publishing. eMIMS. Available from: http://www.emims.net
- Department of Health. The NHS Improvement Plan. Cm 6268. London: Stationery Office, 2004.
- Murphy E. Case management and community matrons for long term conditions. BMJ 2004;329:1251–2.
- Department of Health. Supporting People with Long Term Conditions. An NHS and Social Care Model to Support Local Innovation and Integration. January 2005. Available from: www.dh.gov.uk [accessed 17/01/08].
- Rawlins DR, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224–7.
- Weintraub WS, Zhang Z, Mahoney EM et al. Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation2005;111:1106–13.
- Lee WC, Chavez YE, Baker T, Luce BR. Economic burden of heart failure: a summary of recent literature. Heart Lung 2004;33:362–71.
