Age-related macular degeneration (AMD) is a common ocular condition that may destroy central vision and has a devastating effect on the patient’s quality of life. More than eight million Americans, particularly those over the age of 55 years, suffer from age-related macular degeneration, and the overall prevalence of advanced AMD is projected to increase by more than 50% by the year 2030.1 In the UK, the annual incidence of neovascular AMD was calculated to be around 24,000 in 2005, with a prevalence of 243,000; this is predicted to rise to over 300,000 by 2025.2 The majority of patients with neovascular AMD progress to legal blindness in the affected eye within two years of diagnosis, and there is a 43% probability of progression to neovascular AMD in the other eye within five years.1 Until recently, the only pharmacological-based therapy for treatment of patients with neovascular degeneration has been photodynamic therapy with verteporfin.
Although the pathophysiology is still poorly understood, it is increasingly clear that vascular endothelial growth factor (VEGF) plays an important role in promotion of the neovascularisation and vessel leakage that lead to loss of central vision. Therefore, intravitreal antiangiogenic therapy (injection of antiangiogenic agents directly into the vitreous) is currently the primary therapy for neovascular AMD. Currently, the most common therapeutic agents are ranibizumab, pegaptanib and bevacizumab (used off-label). Anti-VEGF agents administered systemically for other indications in oncology have been associated with serious systemic adverse events and death.3 Since breakdown of the blood-ocular barrier is common in wet AMD, repeated intravitreal anti-VEGF therapy may lead to a small amount of systemic VEGF inhibition, possibly resulting in serious long-term adverse events, though these have not yet been shown in clinical studies.4 We here review the pathogenesis of the disease, the therapeutic options currently used in clinical practice and the possible safety concerns about anti-VEGF therapy in patients with neovascular AMD.
Methods
The leading journals that publish basic science and clinical research in the area of cardiovascular and ophthalmological diseases, and MEDLINE using PubMed, were scanned. The main terms used were “age-related macular degeneration”, “cardiovascular disease”, “ranibizumab”, “bevacizumab”, “pegaptanib” and “VEGF”. The publications were largely selected from the past five years, but older publications which are commonly referenced or highly regarded were not excluded. The reference lists of articles identified by this search strategy were screened and relevant articles were selected. Review articles are cited to provide the reader with information and references beyond the scope of this review.
Age-related macular degeneration
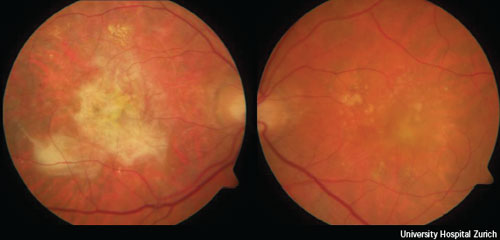
Early age-related macular degeneration is characterised by the presence of a few (< 20) medium-size drusen or retinal pigmentary abnormalities. Intermediate age-related macular degeneration is characterised by at least one large druse, numerous medium-size drusen, or by geographic atrophy that does not extend to the centre of the macula. As reviewed recently by de Jong,5 damage to the retinal pigment epithelium and a chronic aberrant inflammatory response can lead to large areas of retinal atrophy (called geographic atrophy), the expression of angiogenic cytokines such as vascular endothelial growth factor, or both. These processes may manifest as advanced AMD, which can either be non-neovascular (dry, atrophic or non-exudative) or neovascular (wet or exudative). Advanced non-neovascular AMD is characterised by drusen and geographic atrophy extending to the centre of the macula. Advanced neovascular AMD is characterised by choroidal neovascularisation and its sequelae.6 Figure 1 shows the fundus image of a patient with a large fibrosis, the end stage of neovascular AMD, in one eye and recent onset of neovascular AMD in the second eye.
Risk factors for AMD
Several risk factors for the development and progression of AMD have been established in recent years, including advanced age, white race, heredity and a history of smoking. The prevalence of early AMD has been reported to increase from 8% among people 43-54 years of age to 30% among people 75 years or older. Similarly, the prevalence of advanced AMD increases from 0.1% among people 43-54 years of age to 7.1% among people 75 years or older. AMD is more common in whites than Hispanics or Asian persons, whereas blacks have the lowest prevalence of the disease.7 Data from the Human Genome Project revealed that polymorphisms in the complement factor H (CFH), complement factor B (CFB) and the complement component C2 genes may account for 75% of AMD cases.8 A polymorphism (Ala69Sr) on the age-related maculopathy susceptibility 2 gene (ARMS2 or LOC387715) has also been strongly associated with development of AMD.9
Patients with a history of more than 10 pack-years of smoking have an increased risk for the development of AMD and even passive smokers also appear to have a doubled risk of AMD.10 Other modifiable risk factors for advanced AMD include arterial hypertension,11 obesity,12 high dietary fat intake13 and low plasma concentrations of antioxidants and zinc.14,15
Cardiovascular risk factors
Coronary heart disease (CHD) is the leading cause of mortality in the US and Europe for both men and women. Although in England and Wales mortality rates of CHD continue to fall among older age groups, the actual burden of coronary heart disease is increasing due to the ageing of the population.16 The rate of improvement in CHD mortality appears to be declining, and may even be reversing among younger women.16 Atherosclerosis is the underlying cause of most ischaemic events and can result in angina, myocardial infarction, congestive heart failure, cardiac arrhythmias or sudden cardiac death. Risk factors for cardiovascular disease (CVD) have been extensively reviewed in numerous publications.17-19 Data from the INTERHEART study revealed that the major cardiovascular risk factors smoking, hypertension, hypercholesterolaemia, diabetes mellitus, abdominal obesity, sedentary lifestyle and several psychosocial factors account for >92% of cardiovascular events worldwide.19 In the same study, daily consumption of fruits and vegetables, regular alcohol consumption and regular physical activity were associated with a reduced cardiovascular mortality. Most important, these findings were noted in men and women, in old and young people, and all over the world.
Risk factors associated with CVD and AMD
As early as the 1970s, researchers wondered whether AMD might be part of an underlying systemic vascular process or a result of factors that also influence the development of CVD.20,21 The Framingham Eye Study found an association between AMD and systemic blood pressure and its sequel left ventricular hypertrophy.21 The NHANES-I study reported a positive association between AMD and systemic hypertension, and between AMD and cerebrovascular disease.22In a large population-based study, the odds ratio of carotid artery plaques was 4.7 times higher in patients with wet AMD, while peripheral arterial disease was associated with a 2.5 times increased risk for AMD.23
The potential link between AMD and CVD was highlighted further in two recent large US studies. The Atherosclerosis Risk in Communities (ARIC) study in more than 10,000 patients demonstrated that subjects with late AMD were significantly more likely to be diagnosed with incident coronary heart disease over 10 years than patients without late AMD (30.9% vs. 10.0%, respectively),24 and also showed a higher incidence of stroke (4.1% vs. 2.1%).25 The US Medicare Study, a population-based cross-sectional and cohort study involving more than 1.5 million Medicare enrolees ≥ 65 years, found a 20% increased risk of incidental myocardial infarction in patients with neovascular AMD.26Importantly, the Blue Mountains Eye Study, which included more than 3,600 baseline participants in a population-based cohort study of common eye diseases in an Australian population aged ≥ 49 years of age, demonstrated that early AMD predicted a doubling of cardiovascular mortality (relative risk [RR] 2.3, 95% confidence intervals [CI] 1.03 – 5.19) over the next decade after controlling for traditional risk factors.27 Late AMD predicted five-fold higher cardiovascular mortality (RR 5.57, 95% CI 1.35 – 22.99) and ten-fold higher stroke mortality (RR 10.21, 95% CI 2.39 – 43.6) after adjusting for age and gender only.27 Other studies, however, have found no relationship between AMD and CVD.28-34
A potential link between AMD and cardiovascular disease would have important therapeutic implications given current concern that some intravitreal anti-VEGF treatments for wet AMD could increase cardiovascular, and particularly cerebrovascular, risk.35
Indeed, VEGF may be regarded as a double-edged sword. Although it is key in the pathogenesis of wet AMD, it at the same time plays a pivotal role in maintaining vascular integrity, particularly under conditions of ischaemia and hypoxia. Thus, any beneficial effects of anti-VEGF therapies in the eye must be weighed against potential long-term systemic effects of these agents, particularly when potent “pan”-anti-VEGF therapies such as ranibizumab and bevacizumab may exert unwanted systemic extra-ocular effects due to the blocking of the cardioprotective functions of VEGF.
The endothelium is increasingly recognised not only as a target (with vascular remodelling occurring in response to an injury and resulting in atherosclerosis), but as a mediator in the pathogenesis of vascular damage.36 Indeed, endothelial cells play an important homeostatic role in the cardiovascular system through the expression of numerous molecules and release of mediators such as nitric oxide (NO), superoxide and endothelin-1 (ET-1). Studies demonstrating dysfunction of these mediators in patients at risk or with fully developed forms of cardiovascular disease strongly suggest involvement of endothelium-derived factors in the pathogenesis of atherosclerosis and its sequelae.
Functional alterations of the endothelial L-arginine / NO pathway may be important in cardiovascular disease since NO can inhibit substantially several components of the atherogenic process such as vascular smooth muscle cell contraction, proliferation and migration; platelet aggregation and adhesion; monocyte adhesion and oxidative modification of low-density lipoprotein (LDL). Hence, reduced endothelial NO release may accelerate the progression of atherosclerotic lesions. Most importantly, NO is the downstream mediator of VEGF and is considered an important defence system in maintaining vascular integrity.37
VEGF was first described as a tumour-derived factor with potent ability to induce endothelial cell permeability,38 proliferation and angiogenesis.39,40 VEGF induces angiogenesis, and increases vascular permeability and inflammation: all of these are thought to contribute to the progression of the neovascular form of AMD. VEGF levels are raised in the retinal pigment epithelium and choroidal blood vessels of the macula and in the ocular fluid of most patients with proliferative diabetic retinopathy and retinal vein occlusion.
While VEGF occurs in several biologically active forms, a recombinant, humanised monoclonal antibody fragment (Fab), ranibizumab, neutralises all forms of the growth factor. In 2006, two trials with ranibizumab showed that monthly intravitreal injections prevented vision loss and, in many cases, significantly improved the visual acuity of patients with neovascular AMD.41,42 Bevacizumab is a full-length monoclonal antibody that – like ranibizumab – binds and inhibits all isoforms of VEGF, but with a lower affinity, and has a longer half-life compared to the fragment form.

The Food and Drugs Administration (FDA) approved intravenous bevacizumab for patients with metastatic colorectal cancer in February 2004. The same year the first use of systemic bevacizumab for mostly bilateral neovascular AMD was reported.43 Rapid regression of choroidal neovascularisation was associated with a visual acuity improvement of 1-2 lines. A common adverse effect was an increase in systolic blood pressure.44,45 The studies were too small, however, to exclude other serious systemic complications, including increased risk of thromboembolic events, haemorrhage, proteinuria, wound healing complications, and gastro-intestinal perforation.46 In 2005 the first report on the intravitreal use of bevacizumab in neovascular AMD was reported.47 The driving force for the “off-label” use of bevacizumab was, in addition to its obvious clinical effectiveness (figure 2), its low price of less than 50 USD per intravitreal injection. In the meantime, several retrospective and prospective studies indicate good functional outcomes.48-52
So far there has been no evidence for an increased risk for systemic adverse events, but studies are overall too small and follow-up is too short to fully evaluate potential systemic adverse events. Recent meta-analyses indicated comparable functional outcomes and safety with bevacizumab and ranibizumab.53,54 Several large prospective randomised clinical trials comparing bevacizumab and ranibizumab are currently ongoing. Intravenous use of bevacizumab in cancer patients may have serious systemic complications, including increased risk of thromboembolic events, hypertension, haemorrhage, proteinuria, wound healing complications and gastro-intestinal perforation.55 Whether these systemic complications are relevant to wet AMD patients receiving very low doses by intravitreal injection is unknown. The absence of systemic and ocular adverse events in prospective studies is reasssuring, but the long-term safety of intravitreal bevacizumab remains to be established.48,50,52
Pegaptanib was granted marketing authorisation by the European Medicines Agency on 31 January 2006 for the treatment of neovascular AMD. Pegaptanib is a pegylated modified oligonucleotide that binds with high specificity and affinity to extracellular vascular endothelial growth factor (VEGF165), inhibiting its activity. VEGF165 is the VEGF isoform preferentially involved in pathological ocular neovascularisation. Pegaptanib blocks mainly VEGF165, reducing the growth of pathological blood vessels and associated bleeding and leakage.
The main prospective clinical trials in patients with AMD have been conducted using pegaptanib and ranibizumab.41,42,56,57 Indirect comparison of pegaptanib and ranibizumab, indicates about a 3-line difference in visual acuity outcomes in favour of ranibizumab. Pegaptanib appears to be associated with fewer adverse effects although a direct comparison has never been studied. The three pivotal studies on ranibizumab reported a dose-related increased frequency of cardiovascular events (including stroke) and bleeding relative to the placebo group, although these increases were not statistically significant.
Indeed, whilst these new treatments represent an important breakthrough in wet AMD management, the overall safety of intravitreal anti-VEGF drugs remains unclear. First, although the drug is administered by injection through the sclera into the vitreous cavity, systemic absorption does occur, with potential for systemic adverse effects. In particular, human data are scant. Most importantly, since anti-VEGF treatment is potentially required for years, chronic treatment, particularly with non-selective VEGF inhibitors, may cause adverse effects that may only become clinically apparent over time. Second, because these trials were not designed to detect small differences in risk, much larger cohorts would be necessary to allow the evaluation of systemic adverse effects. In the reported clinical studies, the overall mortality rates were low given the advanced mean age of these populations (nearly 80 years); this could be mainly due to the exclusion of patients with a history of, or with risk factors for, cardiovascular disease.
Potential implications in neovascular AMD
It is of note that VEGF, mostly through its downstream mediator NO, has many essential physiological functions in maintaining vascular integrity, including the potential formation of collateral vessels crucial for the maintenance of perfusion to ischaemic tissues, as in acute myocardial infarction, in particular.58 Intriguingly, while VEGF is crucial in maintaining vascular homeostasis, particular in clinical conditions associated with ischaemia and hypoxia, its role in maintaining plaque stability is currently a matter of debate.59 Conceptually, in view of the cardioprotective role of VEGF, non-selective “pan”-anti-VEGF antagonism with ranibizumab or bevacizumab could be of even greater concern than blocking VEGF with selective antagonists such as pegaptanib. However, whether and to what degree more selective VEGF inhibition translates into fewer unwanted systemic effects and thus results in better cardiovascular outcomes remains unproven.
It is of note that cardiovascular safety of anti-VEGF drugs has not yet been addressed in randomised clinical trials. Unfortunately, the numbers of cardiovascular events in these AMD trials without pre-specified cardiovascular safety end points are too small to provide any clinically relevant evidence of safety. Indeed, the absence of evidence does not show evidence of absence. While trials for ranibizumab reported a marginally higher rate of arterial thromboembolic events in the higher dose treatment arm, this trend did not reach statistical significance.60 (These trials were not powered to detect small differences in risk.) The issue is complicated further by a recent retrospective reanalysis of systemic safety outcomes with ranibizumab, which did not use the full dataset but nevertheless showed a significant increase in non-ocular haemorrhage in treated patients compared with controls (p=0.01), suggesting some impairment of systemic VEGF function.61 Although the doses involved in intravitreal injections of anti-VEGF agents are smaller than intravenous doses, intravitreal injection leads to peak serum concentrations several orders of magnitude greater than physiological levels of VEGF (11–27 ng/ml vs. 100 pg/ml in healthy adults).62,63 The potential capacity of both drugs to saturate circulating VEGF hints at the possibility of adverse systemic effects.
Trials with the only selective inhibitor, pegaptanib, have not as yet shown any cardiovascular safety signals.56 Importantly, however, all trials both with less selective or “pan”-anti-VEGF agents were underpowered and thus it is not possible to rule out existing cardiovascular safety concerns. Uncertainty about the cardiovascular risk of intravitreal anti-VEGF treatment will remain until additional systemic safety data become available. These adverse events may be explained by endothelial dysfunction induced by anti-VEGF drugs.
Pan anti-VEGF therapy has shown to stabilise vision in about 95% of patients with neovascular macular degeneration compared to 70% with selective VEGF inhibition. The results are most impressive compared to laser led photocoagulation 15 years ago, which led to an unselective destruction of the retina and a primary loss of vision in order to stop progression of neovascular AMD. This obvious benefit from an ophthalmological perspective stands in contrast to potential cardiovascular risks. Only adequately powered randomised clinical trials that prospectively address cardiovascular safety will provide the evidence to show whether the proven benefits of VEGF antagonism in the eye may come at the cost of potential systemic adverse effects, particularly increasing atherosclerosis and its clinical sequelae. Until this trial evidence becomes available, ophthalmologists should avoid continuous VEGF suppression by using individualised treatment protocols64 and should synchronise their efforts with cardiologists to reduce the cardiovascular burden of patients with wet AMD.
Conflict of interest
None declared.
References
- Friedman DS, O’Colmain BJ, Munoz B et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72.
- Lotery A, Xu X, Zlatava G, Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol 2007;91:1303–07.
- Pereg D, Lishner M. Bevacizumab treatment for cancer patients with cardiovascular disease: a double edged sword? Eur Heart J 2008;29:2325–6.
- van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA 2005;293:1509–13.
- de Jong PT. Age-related macular degeneration. N Engl J Med 2006;355:1474–85.
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358:2606–17.
- Klein R, Klein BE, Knudtson MD et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006;113:373-80.
- Gold B, Merriam JE, Zernant J et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet 2006;38:458–62.
- Kanda A, Chen W, Othman M et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A 2007;104:16227–32.
- Khan JC, Thurlby DA, Shahid H et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol 2006;90:75–80.
- Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol 1997;115:741-7.
- Schaumberg DA, Christen WG, Hankinson SE, Glynn RJ. Body mass index and the incidence of visually significant age-related maculopathy in men. Arch Ophthalmol 2001;119:1259–65.
- Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003;121:1728–37.
- van Leeuwen R, Boekhoorn S, Vingerling JR et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005;294:3101–07.
- VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol 1998;148:204–14.
- Allender S, Scarborough P, O’Flaherty M, Capewell S. Patterns of coronary heart disease mortality over the 20th century in England and Wales: Possible plateaus in the rate of decline. BMC Public Health 2008;8:148.
- Manson JE, Tosteson H, Ridker PM et al. The primary prevention of myocardial infarction. N Engl J Med 1992;326:1406–16.
- Rich-Edwards JW, Manson JE, Hennekens CH, Buring JE. The primary prevention of coronary heart disease in women. N Engl J Med 1995;332:1758–66.
- Yusuf S, Hawken S, Ounpuu S et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52.
- Hyman LG, Lilienfeld AM, Ferris FL 3rd, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol 1983;118:213–27.
- Kahn HA, Leibowitz HM, Ganley JP et al. The Framingham Eye Study. II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol 1977;106:33–41.
- Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MO. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol1988;128:700–10.
- Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol 1995;142:404–09.
- Wong TY, Tikellis G, Sun C, Klein R, Couper DJ, Sharrett AR. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology 2007;114:86–91.
- Wong TY, Klein R, Sun C et al. Age-related macular degeneration and risk for stroke. Ann Intern Med 2006;145:98–106.
- Duan Y, Mo J, Klein R et al. Age-related macular degeneration is associated with incident myocardial infarction among elderly Americans. Ophthalmology 2007;114:732–7.
- Tan JS, Wang JJ, Liew G, Rochtchina E, Mitchell P. Age-related macular degeneration and mortality from cardiovascular disease or stroke. Br J Ophthalmol 2008;92:509–12.
- Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol 1992;110:1701–08.
- Delaney WV Jr, Oates RP. Senile macular degeneration: a preliminary study. Ann Ophthalmol 1982;14: 21–4.
- 30. Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1993;100: 406–14.
- Maltzman BA, Mulvihill MN, Greenbaum A. Senile macular degeneration and risk factors: a case-control study. Ann Ophthalmol 1979;11:1197–201.
- Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol 1998;116:583–7.
- Vinding T. Age-related macular degeneration. An epidemiological study of 1000 elderly individuals. With reference to prevalence, fundoscopic findings, visual impairment and risk factors. Acta Ophthalmol Scand Suppl 1995:1:32.
- Vinding T, Appleyard M, Nyboe J, Jensen G. Risk factor analysis for atrophic and exudative age-related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 1992;70:66–72.
- Liew G, Mitchell P. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2007;356:747-8; author reply 749–50.
- Lüscher TF VP. The Endothelium: Modulator of Cardiovascular Function. Boca Ranton: CRC Press, 1990.
- El-Remessey AB, Tsai N, Caldwell RB. Is superoxide anion a mediator of vascular endothelial growth factor (VEGF) angiogenic function in retinal endothelial cells? Invest Ophthalmol Vis Sci 2003;44: E-abstract 2913.
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–5.
- Carmeliet P, Ferreira V, Breier G et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–9.
- Ferrara N, Carver-Moore K, Chen H et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–42.
- Rosenfeld PJ, Brown DM, Heier JS et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31.
- Brown DM, Kaiser PK, Michels M et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44.
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study.Ophthalmology 2005;112:1035–47.
- Geitzenauer W, Michels S, Prager F et al. Comparison of 2.5 mg/kg and 5 mg/kg systemic bevacizumab in neovascular age-related macular degeneration: twenty-four week results of an uncontrolled, prospective cohort study. Retina2008;28:1375–86.
- Moshfeghi AA, Rosenfeld PJ, Puliafito CA et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology2006;113:2002 e1-e12.
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005;333:328–35.
- Rosenfeld PJ, Moshfegi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36:331–5.
- Algvere PV, Steen B, Seregard S, Kvanta A. A prospective study on intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration of different durations. Acta Ophthalmol. 2008: 86:482–9.
- Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2006;142:1–9.
- Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin BN. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol 2008;145:249–56.
- Cleary CA, Jungkim S, Ravikumar K, Kelliher C, Acheson RW, Hickey-Dwyer M. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9-month results. Eye 2008;22:82–6.
- Weigert G, Michels S, Sacu S et al. Intravitreal bevacizumab (Avastin) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age-related macular degeneration: 6-month results of a prospective, randomised, controlled clinical study. Br J Ophthalmol 2008;92:356–60.
- Algvere PV, Kvanta A, Seregard S. Shall we use Avastin or Lucentis for ocular neovascularization? Acta Ophthalmol 2008;86:352–5.
- Schouten JS, La Heij EC, Webers CA, Lundqvist IJ, Hendrikse F. A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2009;247:1–11.
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005;333:328–35.
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805–16.
- Heier JS, Boyer DS, Ciulla TA et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol 2006;124:1532–42.
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001;280:C1358–66.
- Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat Clin Pract Cardiovasc Med 2007;4:491–502.
- Summary of product characteristics. http:www.emea.europa.eu/humandocs/PDFs/EPAR/Lucentis/H-715-Pl-en.pdf
- Gillies MC, Wong TY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2007;356:748–9; author reply 749-50.
- Larsson A, Skoldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis 2002;5:107–10.
- Wong TY, Liew G, Mitchell P. Clinical update: new treatments for age-related macular degeneration. Lancet 2007;370:204–6.
- Fung AE, Lalwani GA, Rosenfeld PJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol2007;143:566–83.
